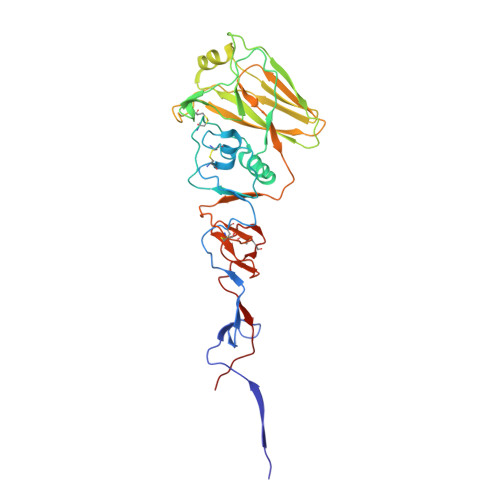
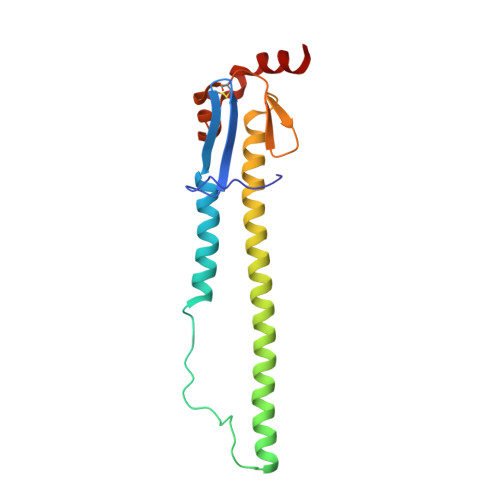
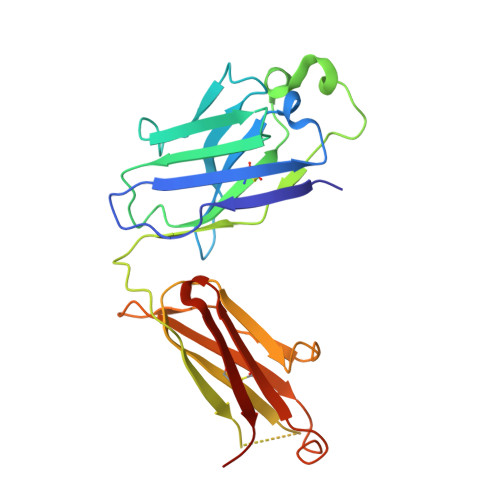
Unique structural solution from a V H 3-30 antibody targeting the hemagglutinin stem of influenza A viruses.
Harshbarger, W.D., Deming, D., Lockbaum, G.J., Attatippaholkun, N., Kamkaew, M., Hou, S., Somasundaran, M., Wang, J.P., Finberg, R.W., Zhu, Q.K., Schiffer, C.A., Marasco, W.A.(2021) Nat Commun 12: 559-559
- PubMed: 33495478
- DOI: https://doi.org/10.1038/s41467-020-20879-6
- Primary Citation of Related Structures:
6WEX, 6WEZ, 6WF0, 6WF1 - PubMed Abstract:
Broadly neutralizing antibodies (bnAbs) targeting conserved influenza A virus (IAV) hemagglutinin (HA) epitopes can provide valuable information for accelerating universal vaccine designs. Here, we report structural details for heterosubtypic recognition of HA from circulating and emerging IAVs by the human antibody 3I14. Somatic hypermutations play a critical role in shaping the HCDR3, which alone and uniquely among V H 3-30 derived antibodies, forms contacts with five sub-pockets within the HA-stem hydrophobic groove. 3I14 light-chain interactions are also key for binding HA and contribute a large buried surface area spanning two HA protomers. Comparison of 3I14 to bnAbs from several defined classes provide insights to the bias selection of V H 3-30 antibodies and reveals that 3I14 represents a novel structural solution within the V H 3-30 repertoire. The structures reported here improve our understanding of cross-group heterosubtypic binding activity, providing the basis for advancing immunogen designs aimed at eliciting a broadly protective response to IAV.
- Department of Cancer Immunology and Virology, Dana-Farber Cancer Institute, Boston, MA, USA.
Organizational Affiliation: