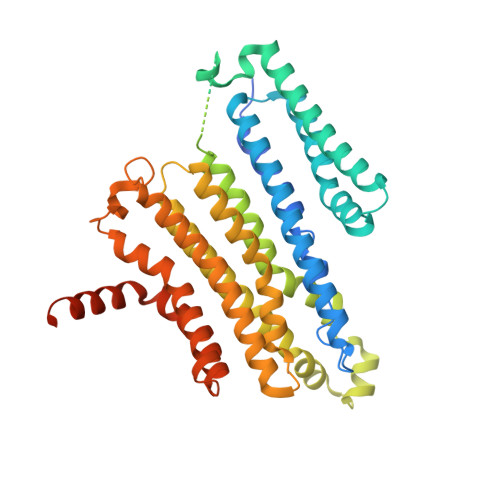
Gating and selectivity mechanisms for the lysosomal K + channel TMEM175.
Oh, S., Paknejad, N., Hite, R.K.(2020) Elife 9
- PubMed: 32228865
- DOI: https://doi.org/10.7554/eLife.53430
- Primary Citation of Related Structures:
6WC9, 6WCA, 6WCB, 6WCC - PubMed Abstract:
Transmembrane protein 175 (TMEM175) is a K + -selective ion channel expressed in lysosomal membranes, where it establishes a membrane potential essential for lysosomal function and its dysregulation is associated with the development of Parkinson's Disease. TMEM175 is evolutionarily distinct from all known channels, predicting novel ion-selectivity and gating mechanisms. Here we present cryo-EM structures of human TMEM175 in open and closed conformations, enabled by resolutions up to 2.6 Å. Human TMEM175 adopts a homodimeric architecture with a central ion-conduction pore lined by the side chains of the pore-lining helices. Conserved isoleucine residues in the center of the pore serve as the gate in the closed conformation. In the widened channel in the open conformation, these same residues establish a constriction essential for K + selectivity. These studies reveal the mechanisms of permeation, selectivity and gating and lay the groundwork for understanding the role of TMEM175 in lysosomal function.
- Structural Biology Program, Memorial Sloan Kettering Cancer Center, New York, United States.
Organizational Affiliation: