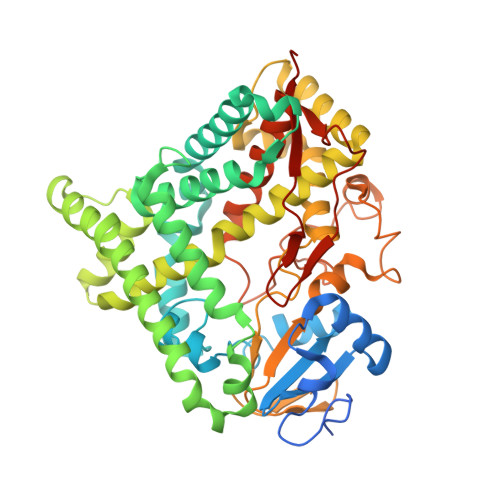
Structure and Function of the Cytochrome P450 Monooxygenase Cinnamate 4-hydroxylase fromSorghum bicolor.
Zhang, B., Lewis, K.M., Abril, A., Davydov, D.R., Vermerris, W., Sattler, S.E., Kang, C.(2020) Plant Physiol 183: 957-973
- PubMed: 32332088
- DOI: https://doi.org/10.1104/pp.20.00406
- Primary Citation of Related Structures:
6VBY - PubMed Abstract:
Cinnamate 4-hydroxylase (C4H; CYP73A) is a cytochrome P450 monooxygenase associated externally with the endoplasmic reticulum of plant cells. The enzyme uses NADPH-cytochrome P450 reductase as a donor of electrons and hydroxylates cinnamic acid to form 4-coumaric acid in phenylpropanoid metabolism. In order to better understand the structure and function of this unique class of plant P450 enzymes, we have characterized the enzyme C4H1 from lignifying tissues of sorghum ( Sorghum bicolor ), encoded by Sobic.002G126600 Here we report the 1.7 Å resolution crystal structure of CYP73A33. The obtained structural information, along with the results of the steady-state kinetic analysis and the absorption spectroscopy titration, displays a high degree of similarity of the structural and functional features of C4H to those of other P450 proteins. Our data also suggest the presence of a putative allosteric substrate-binding site in a hydrophobic pocket on the enzyme surface. In addition, comparing the newly resolved structure with those of well-investigated cytochromes P450 from mammals and bacteria enabled us to identify those residues of critical functional importance and revealed a unique sequence signature that is potentially responsible for substrate specificity and catalytic selectivity of C4H.
- Department of Chemistry, Washington State University, Pullman, Washington 99164.
Organizational Affiliation: