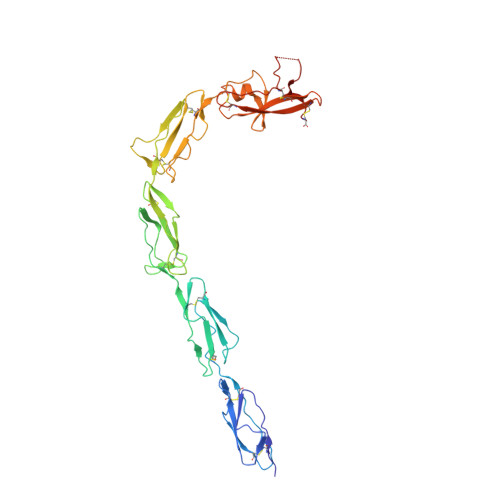
The J-elongated conformation of beta2-glycoprotein I predominates in solution: implications for our understanding of antiphospholipid syndrome.
Ruben, E., Planer, W., Chinnaraj, M., Chen, Z., Zuo, X., Pengo, V., De Filippis, V., Alluri, R.K., McCrae, K.R., Macor, P., Tedesco, F., Pozzi, N.(2020) J Biological Chem 295: 10794-10806
- PubMed: 32518155
- DOI: https://doi.org/10.1074/jbc.RA120.013939
- Primary Citation of Related Structures:
6V06, 6V08, 6V09 - PubMed Abstract:
β 2 -Glycoprotein I (β 2 GPI) is an abundant plasma protein displaying phospholipid-binding properties. Because it binds phospholipids, it is a target of antiphospholipid antibodies (aPLs) in antiphospholipid syndrome (APS), a life-threatening autoimmune thrombotic disease. Indeed, aPLs prefer membrane-bound β 2 GPI to that in solution. β 2 GPI exists in two almost equally populated redox states: oxidized, in which all the disulfide bonds are formed, and reduced, in which one or more disulfide bonds are broken. Furthermore, β 2 GPI can adopt multiple conformations ( i.e. J-elongated, S-twisted, and O-circular). While strong evidence indicates that the J-form is the structure bound to aPLs, which conformation exists and predominates in solution remains controversial, and so is the conformational pathway leading to the bound state. Here, we report that human recombinant β 2 GPI purified under native conditions is oxidized. Moreover, under physiological pH and salt concentrations, this oxidized form adopts a J-elongated, flexible conformation, not circular or twisted, in which the N-terminal domain I (DI) and the C-terminal domain V (DV) are exposed to the solvent. Consistent with this model, binding kinetics and mutagenesis experiments revealed that in solution the J-form interacts with negatively charged liposomes and with MBB2, a monoclonal anti-DI antibody that recapitulates most of the features of pathogenic aPLs. We conclude that the preferential binding of aPLs to phospholipid-bound β 2 GPI arises from the ability of its preexisting J-form to accumulate on the membranes, thereby offering an ideal environment for aPL binding. We propose that targeting the J-form of β 2 GPI provides a strategy to block pathogenic aPLs in APS.
- Edward A. Doisy Department of Biochemistry and Molecular Biology, Saint Louis University School of Medicine, St. Louis, Missouri, USA.
Organizational Affiliation: