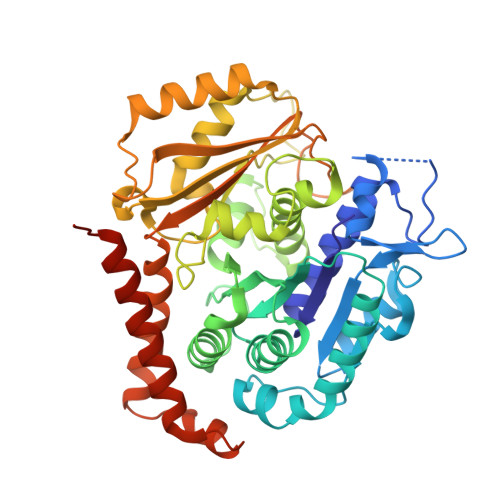
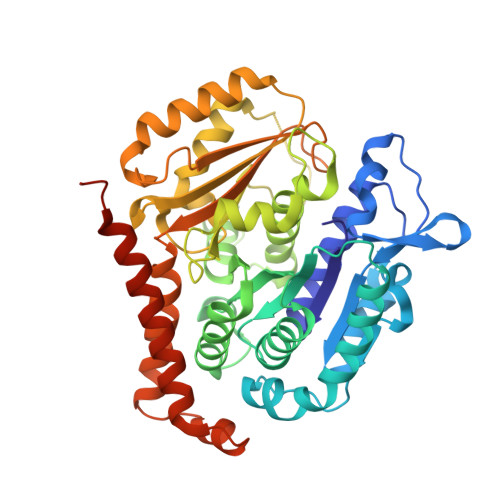
Discovery of dihydrofuranoallocolchicinoids - Highly potent antimitotic agents with low acute toxicity.
Shchegravina, E.S., Svirshchevskaya, E.V., Combes, S., Allegro, D., Barbier, P., Gigant, B., Varela, P.F., Gavryushin, A.E., Kobanova, D.A., Shchekotikhin, A.E., Fedorov, A.Y.(2020) Eur J Med Chem 207: 112724-112724
- PubMed: 32827941
- DOI: https://doi.org/10.1016/j.ejmech.2020.112724
- Primary Citation of Related Structures:
6TDE - PubMed Abstract:
Two series of heterocyclic colchicinoids bearing β-methylenedihydrofuran or 2H-pyran-2-one fragments were synthesized by the intramolecular Heck reaction. Methylenedihydrofuran compounds 9a and 9h were found to be the most cytotoxic among currently known colchicinoids, exhibiting outstanding antiproliferative activity on tumor cell lines in picomolar (0.01-2.1 nM) range of concentrations. Compound 9a potently and substoichiometrically inhibits microtubule formation in vitro, being an order of magnitude more active in this assay than colchicine. Derivatives 9a and 9h revealed relatively low acute toxicity in mice (LD 50 ≥ 10 mg/kg i.v.). The X-Ray structure of colchicinoid 9a bound to tubulin confirmed interaction of this compound with the colchicine binding site of tubulin.
- Department of Organic Chemistry, Nizhny Novgorod State University, Gagarina Av. 23, Nizhny Novgorod, 603950, Russian Federation.
Organizational Affiliation: