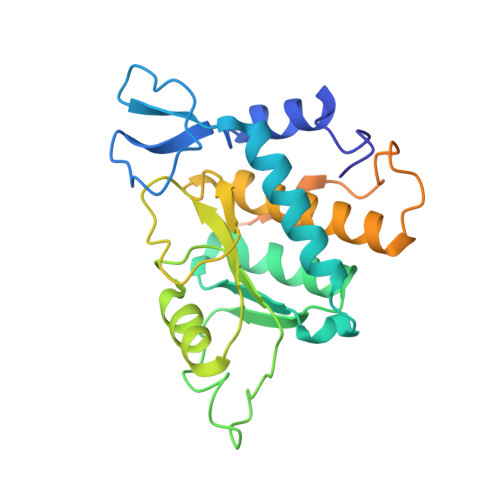
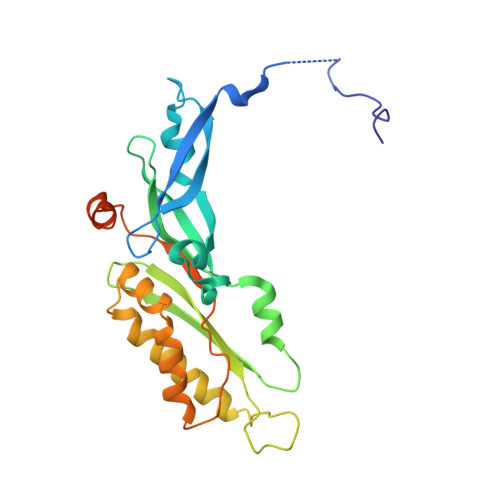
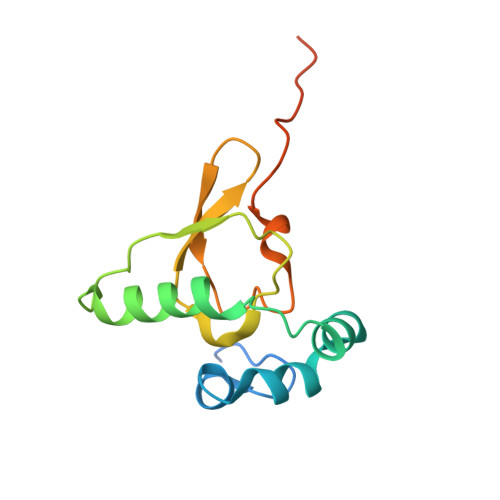
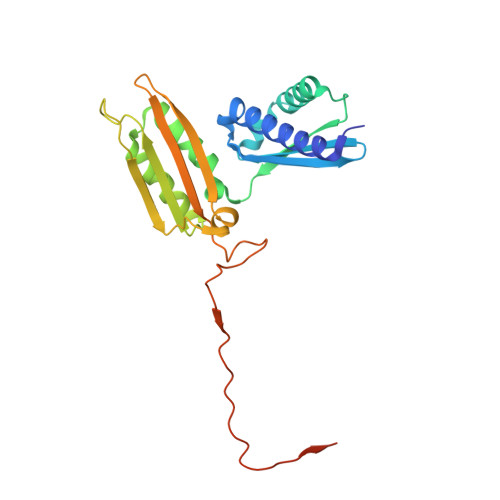
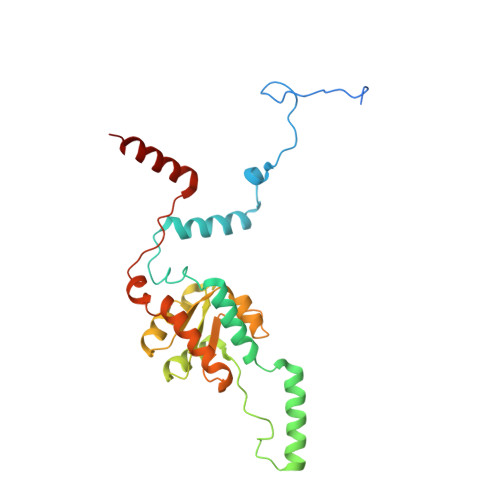
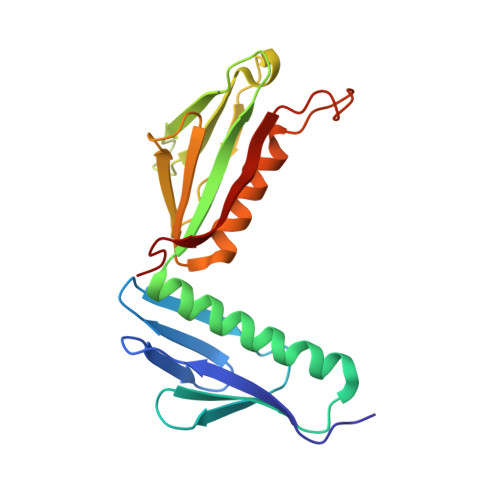
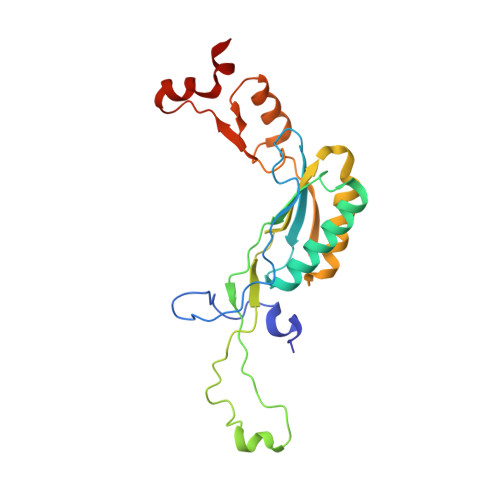
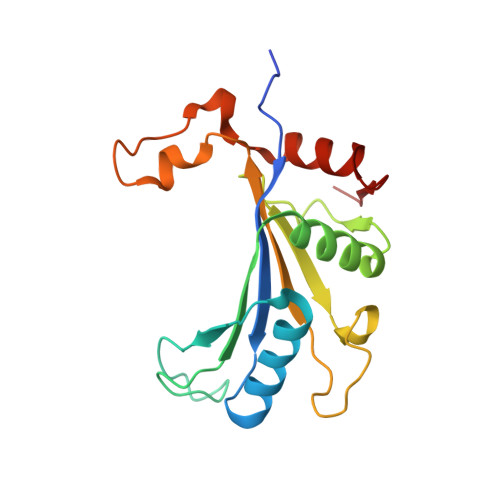
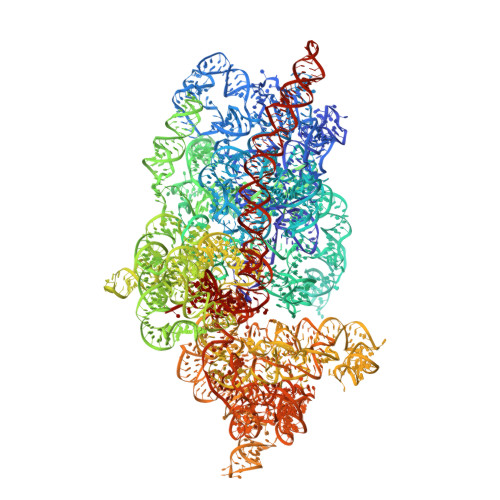
RQT complex dissociates ribosomes collided on endogenous RQC substrate SDD1.
Matsuo, Y., Tesina, P., Nakajima, S., Mizuno, M., Endo, A., Buschauer, R., Cheng, J., Shounai, O., Ikeuchi, K., Saeki, Y., Becker, T., Beckmann, R., Inada, T.(2020) Nat Struct Mol Biol 27: 323-332
- PubMed: 32203490
- DOI: https://doi.org/10.1038/s41594-020-0393-9
- Primary Citation of Related Structures:
6SNT, 6SV4 - PubMed Abstract:
Ribosome-associated quality control (RQC) represents a rescue pathway in eukaryotic cells that is triggered upon translational stalling. Collided ribosomes are recognized for subsequent dissociation followed by degradation of nascent peptides. However, endogenous RQC-inducing sequences and the mechanism underlying the ubiquitin-dependent ribosome dissociation remain poorly understood. Here, we identified SDD1 messenger RNA from Saccharomyces cerevisiae as an endogenous RQC substrate and reveal the mechanism of its mRNA-dependent and nascent peptide-dependent translational stalling. In vitro translation of SDD1 mRNA enabled the reconstitution of Hel2-dependent polyubiquitination of collided disomes and, preferentially, trisomes. The distinct trisome architecture, visualized using cryo-EM, provides the structural basis for the more-efficient recognition by Hel2 compared with that of disomes. Subsequently, the Slh1 helicase subunit of the RQC trigger (RQT) complex preferentially dissociates the first stalled polyubiquitinated ribosome in an ATP-dependent manner. Together, these findings provide fundamental mechanistic insights into RQC and its physiological role in maintaining cellular protein homeostasis.
- Graduate School of Pharmaceutical Sciences, Tohoku University, Sendai, Japan.
Organizational Affiliation: