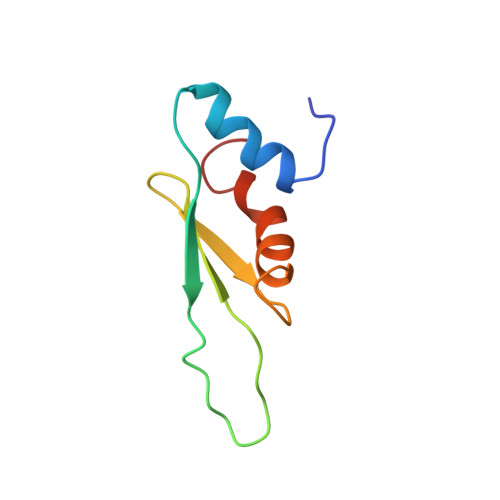
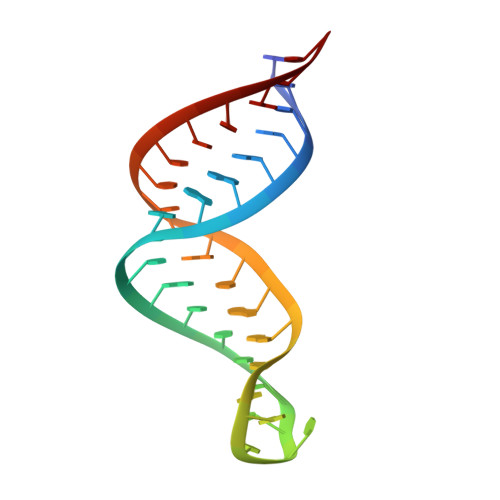
Staufen1 reads out structure and sequence features in ARF1 dsRNA for target recognition.
Yadav, D.K., Zigackova, D., Zlobina, M., Klumpler, T., Beaumont, C., Kubickova, M., Vanacova, S., Lukavsky, P.J.(2020) Nucleic Acids Res 48: 2091-2106
- PubMed: 31875226
- DOI: https://doi.org/10.1093/nar/gkz1163
- Primary Citation of Related Structures:
6SDW, 6SDY - PubMed Abstract:
Staufen1 (STAU1) is a dsRNA binding protein mediating mRNA transport and localization, translational control and STAU1-mediated mRNA decay (SMD). The STAU1 binding site (SBS) within human ADP-ribosylation factor1 (ARF1) 3'UTR binds STAU1 and this downregulates ARF1 cytoplasmic mRNA levels by SMD. However, how STAU1 recognizes specific mRNA targets is still under debate. Our structure of the ARF1 SBS-STAU1 complex uncovers target recognition by STAU1. STAU1 dsRNA binding domain (dsRBD) 4 interacts with two pyrimidines and one purine from the minor groove side via helix α1, the β1-β2 loop anchors the dsRBD at the end of the dsRNA and lysines in helix α2 bind to the phosphodiester backbone from the major groove side. STAU1 dsRBD3 displays the same binding mode with specific recognition of one guanine base. Mutants disrupting minor groove recognition of ARF1 SBS affect in vitro binding and reduce SMD in vivo. Our data thus reveal how STAU1 recognizes minor groove features in dsRNA relevant for target selection.
- Central European Institute of Technology, Masaryk University, Kamenice 753/5, 62500, Brno, Czech Republic.
Organizational Affiliation: