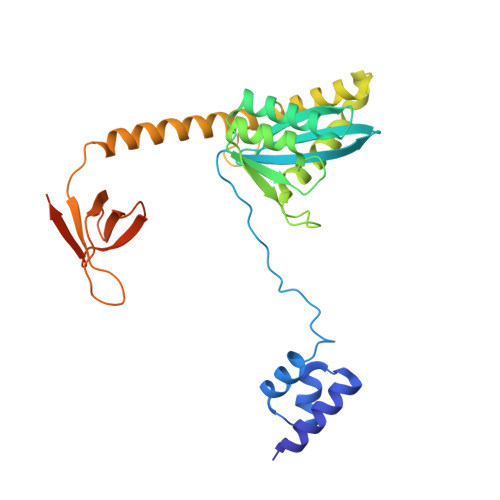
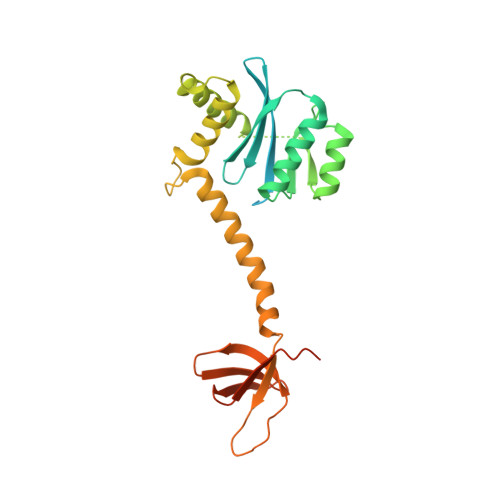
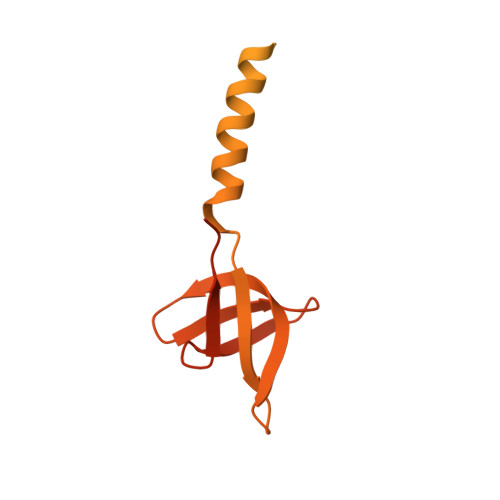
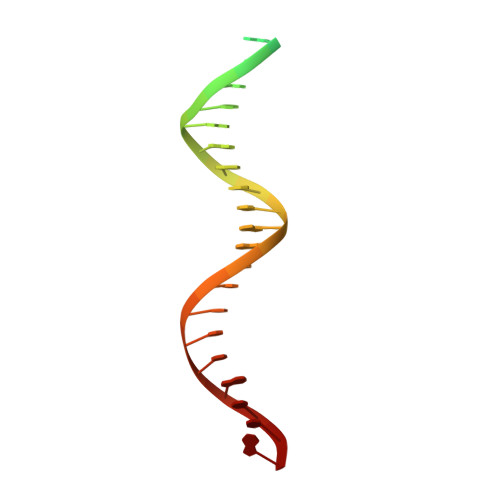
Structural basis of second-generation HIV integrase inhibitor action and viral resistance.
Cook, N.J., Li, W., Berta, D., Badaoui, M., Ballandras-Colas, A., Nans, A., Kotecha, A., Rosta, E., Engelman, A.N., Cherepanov, P.(2020) Science 367: 806-810
- PubMed: 32001525
- DOI: https://doi.org/10.1126/science.aay4919
- Primary Citation of Related Structures:
6RWL, 6RWM, 6RWN, 6RWO - PubMed Abstract:
Although second-generation HIV integrase strand-transfer inhibitors (INSTIs) are prescribed throughout the world, the mechanistic basis for the superiority of these drugs is poorly understood. We used single-particle cryo-electron microscopy to visualize the mode of action of the advanced INSTIs dolutegravir and bictegravir at near-atomic resolution. Glutamine-148→histidine (Q148H) and glycine-140→serine (G140S) amino acid substitutions in integrase that result in clinical INSTI failure perturb optimal magnesium ion coordination in the enzyme active site. The expanded chemical scaffolds of second-generation compounds mediate interactions with the protein backbone that are critical for antagonizing viruses containing the Q148H and G140S mutations. Our results reveal that binding to magnesium ions underpins a fundamental weakness of the INSTI pharmacophore that is exploited by the virus to engender resistance and provide a structural framework for the development of this class of anti-HIV/AIDS therapeutics.
- Chromatin Structure and Mobile DNA Laboratory, Francis Crick Institute, London NW1 1AT, UK.
Organizational Affiliation: