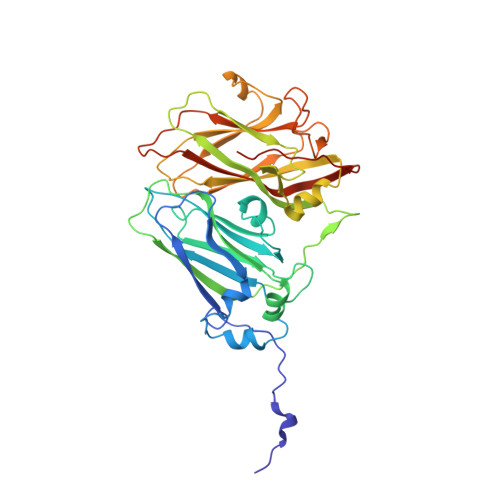
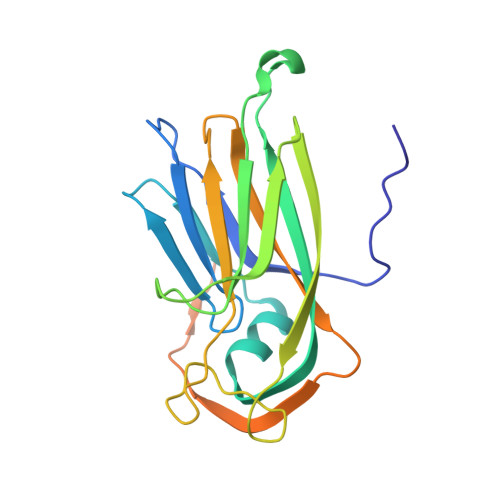
The cryo-electron microscopy structure of Broad Bean Stain Virus suggests a common capsid assembly mechanism among comoviruses.
Lecorre, F., Lai-Kee-Him, J., Blanc, S., Zeddam, J.L., Trapani, S., Bron, P.(2019) Virology 530: 75-84
- PubMed: 30782565
- DOI: https://doi.org/10.1016/j.virol.2019.02.009
- Primary Citation of Related Structures:
6QCC - PubMed Abstract:
The Broad bean stain virus (BBSV) is a member of the genus Comovirus infecting Fabaceae. The virus is transmitted through seed and by plant weevils causing severe and widespread disease worldwide. BBSV has a bipartite, positive-sense, single-stranded RNA genome encapsidated in icosahedral particles. We present here the cryo-electron microscopy reconstruction of the BBSV and an atomic model of the capsid proteins refined at 3.22 Å resolution. We identified residues involved in RNA/capsid interactions revealing a unique RNA genome organization. Inspection of the small coat protein C-terminal domain highlights a maturation cleavage between Leu567 and Leu568 and interactions of the C-terminal stretch with neighbouring small coat proteins within the capsid pentameric turrets. These interactions previously proposed to play a key role in the assembly of the Cowpea mosaic virus suggest a common capsid assembly mechanism throughout all comovirus species.
- Centre de Biochimie Structurale (CBS), INSERM, CNRS, Univ. Montpellier, 29 rue de Navacelles, 34090 Montpellier, France.
Organizational Affiliation: