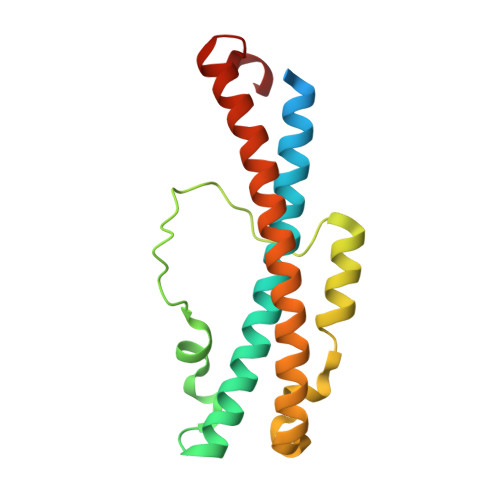
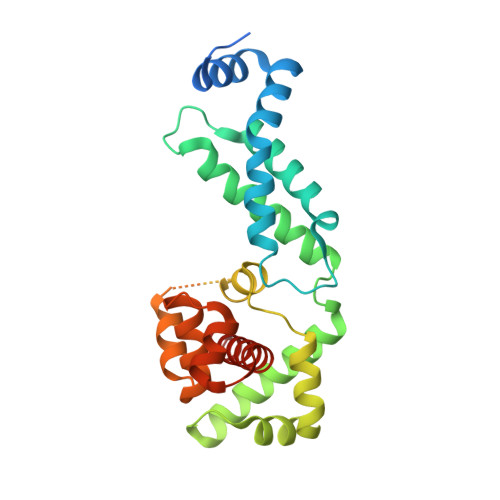
c-di-GMP Arms an Anti-sigma to Control Progression of Multicellular Differentiation in Streptomyces.
Gallagher, K.A., Schumacher, M.A., Bush, M.J., Bibb, M.J., Chandra, G., Holmes, N.A., Zeng, W., Henderson, M., Zhang, H., Findlay, K.C., Brennan, R.G., Buttner, M.J.(2020) Mol Cell 77: 586
- PubMed: 31810759
- DOI: https://doi.org/10.1016/j.molcel.2019.11.006
- Primary Citation of Related Structures:
6PFJ, 6PFV - PubMed Abstract:
Streptomyces are our primary source of antibiotics, produced concomitantly with the transition from vegetative growth to sporulation in a complex developmental life cycle. We previously showed that the signaling molecule c-di-GMP binds BldD, a master repressor, to control initiation of development. Here we demonstrate that c-di-GMP also intervenes later in development to control differentiation of the reproductive hyphae into spores by arming a novel anti-σ (RsiG) to bind and sequester a sporulation-specific σ factor (σ WhiG ). We present the structure of the RsiG-(c-di-GMP) 2 -σ WhiG complex, revealing an unusual, partially intercalated c-di-GMP dimer bound at the RsiG-σ WhiG interface. RsiG binds c-di-GMP in the absence of σ WhiG , employing a novel E(X) 3 S(X) 2 R(X) 3 Q(X) 3 D motif repeated on each helix of a coiled coil. Further studies demonstrate that c-di-GMP is essential for RsiG to inhibit σ WhiG . These findings reveal a newly described control mechanism for σ-anti-σ complex formation and establish c-di-GMP as the central integrator of Streptomyces development.
- Department of Molecular Microbiology, John Innes Centre, Norwich Research Park, Norwich NR4 7UH, UK.
Organizational Affiliation: