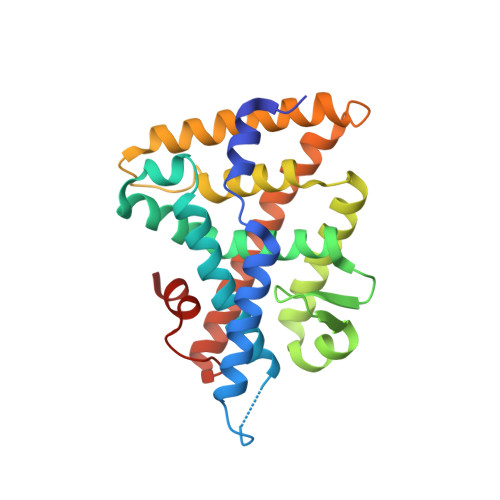
Development of the First Low Nanomolar Liver Receptor Homolog-1 Agonist through Structure-guided Design.
Mays, S.G., Flynn, A.R., Cornelison, J.L., Okafor, C.D., Wang, H., Wang, G., Huang, X., Donaldson, H.N., Millings, E.J., Polavarapu, R., Moore, D.D., Calvert, J.W., Jui, N.T., Ortlund, E.A.(2019) J Med Chem 62: 11022-11034
- PubMed: 31419141
- DOI: https://doi.org/10.1021/acs.jmedchem.9b00753
- Primary Citation of Related Structures:
6OQX, 6OQY, 6OR1 - PubMed Abstract:
As a key regulator of metabolism and inflammation, the orphan nuclear hormone receptor, liver receptor homolog-1 (LRH-1), has potential as a therapeutic target for diabetes, nonalcoholic fatty liver disease, and inflammatory bowel diseases (IBD). Discovery of LRH-1 modulators has been difficult, in part due to the tendency for synthetic compounds to bind unpredictably within the lipophilic binding pocket. Using a structure-guided approach, we exploited a newly discovered polar interaction to lock agonists in a consistent orientation. This enabled the discovery of the first low nanomolar LRH-1 agonist, one hundred times more potent than the best previous modulator. We elucidate a novel mechanism of action that relies upon specific polar interactions deep in the LRH-1 binding pocket. In an organoid model of IBD, the new agonist increases expression of LRH-1-controlled steroidogenic genes and promotes anti-inflammatory gene expression changes. These studies constitute major progress in developing LRH-1 modulators with potential clinical utility.
- Department of Biochemistry , Emory University School of Medicine , Atlanta , Georgia 30322 , United States.
Organizational Affiliation: