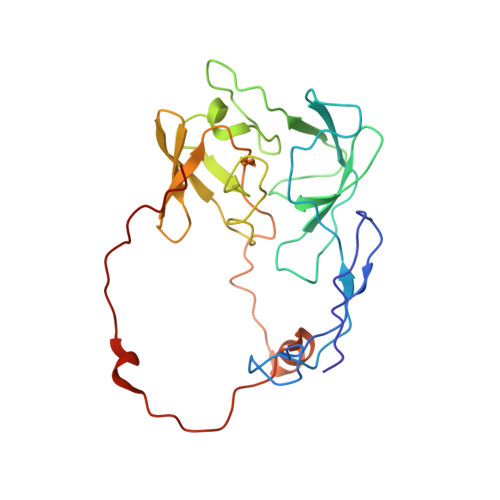
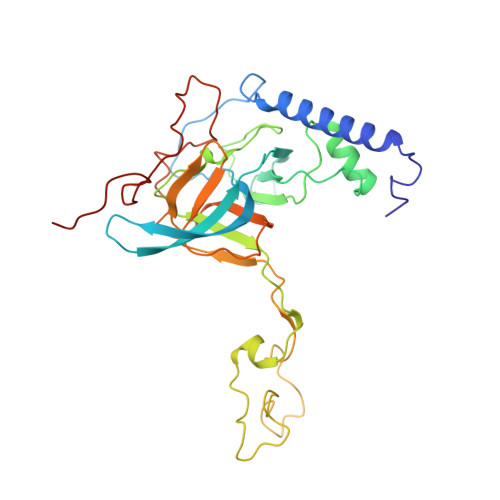
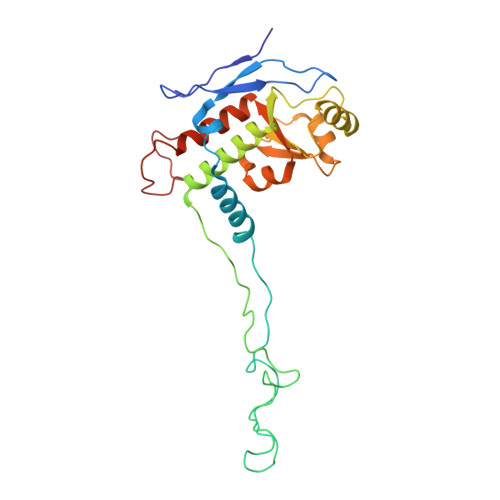
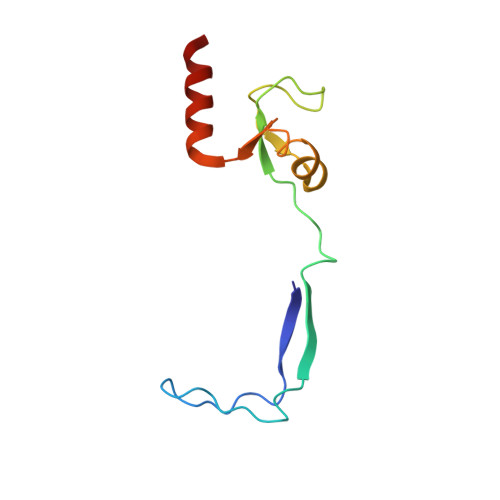
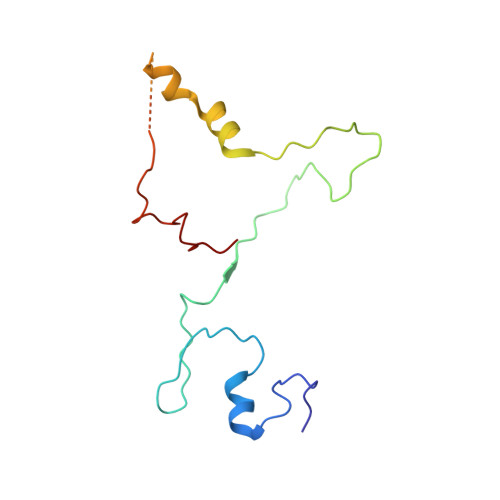
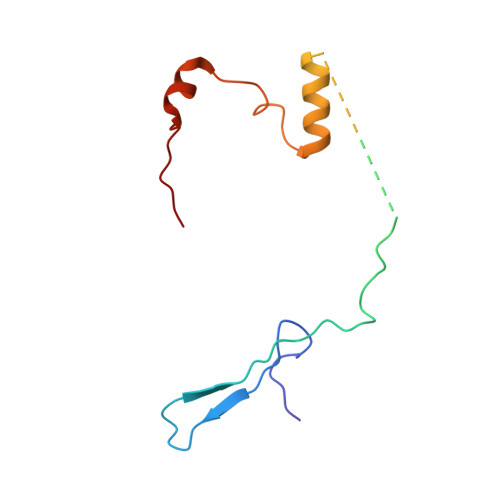
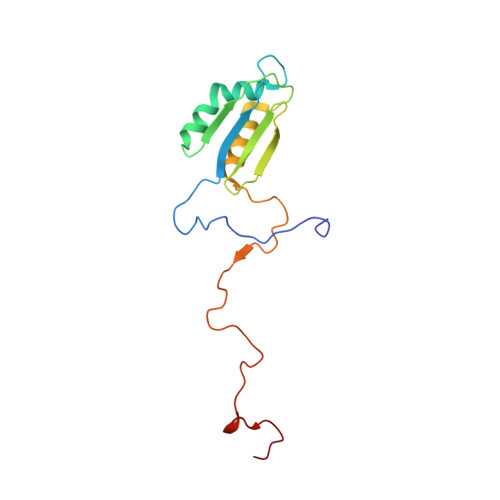
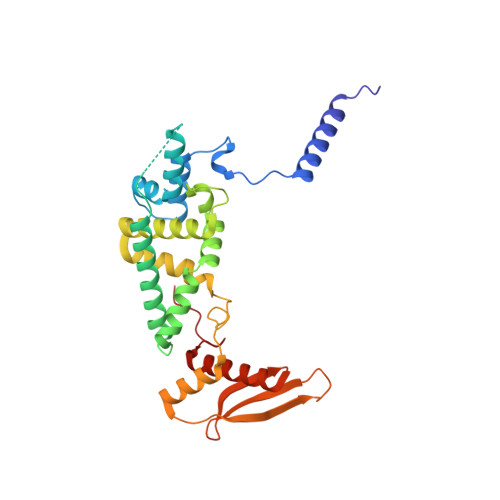
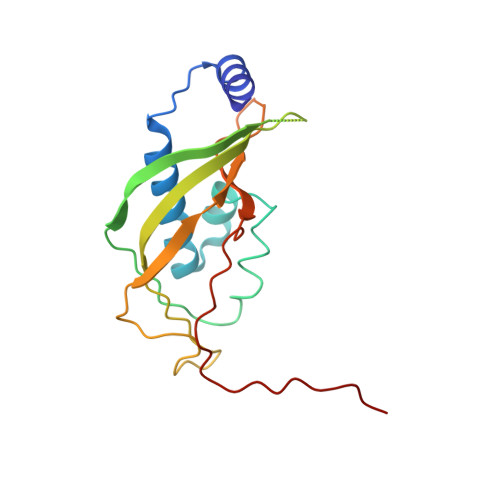
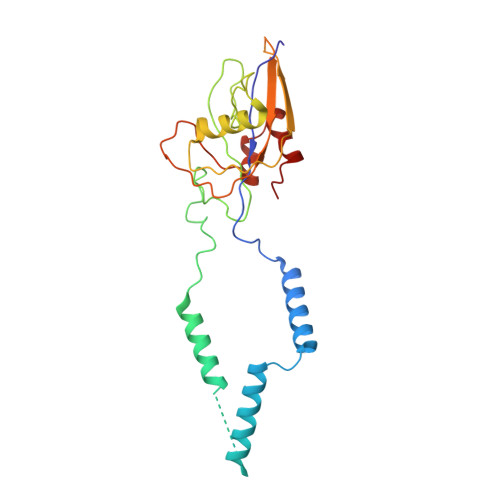
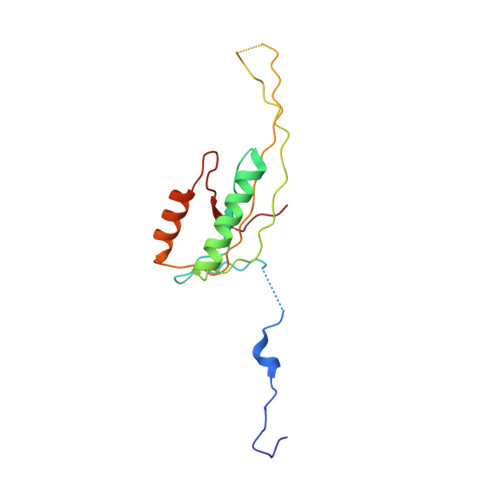
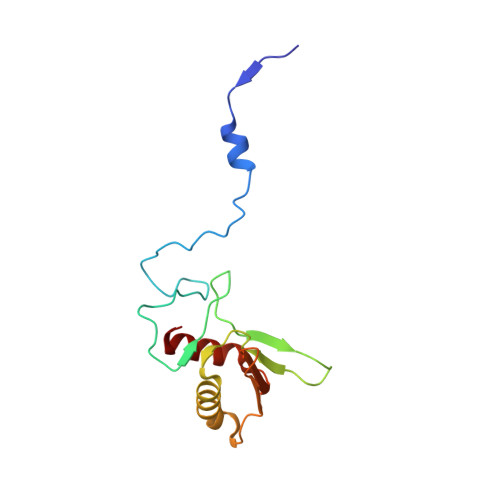
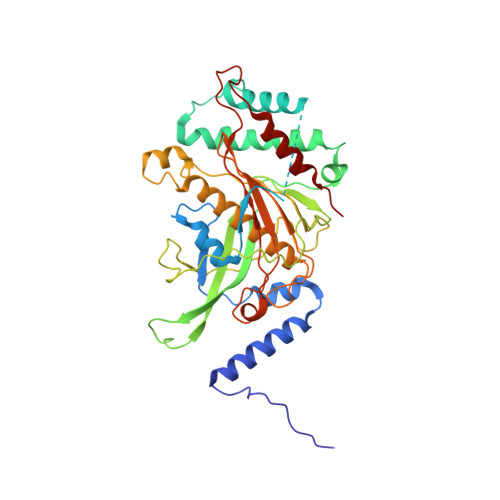
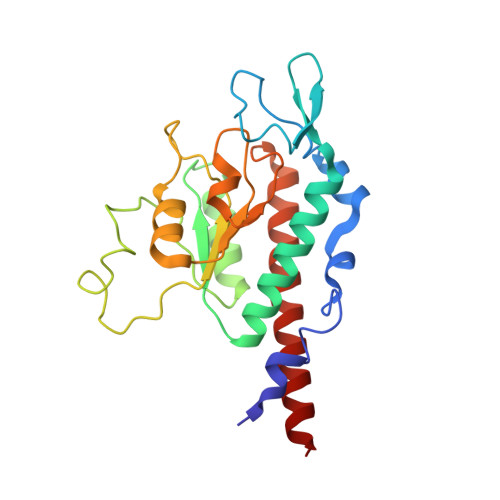
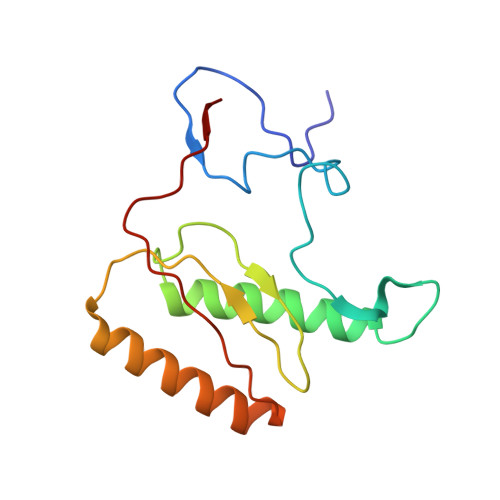
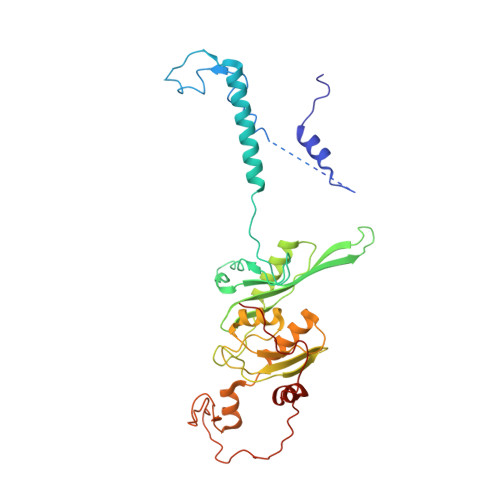
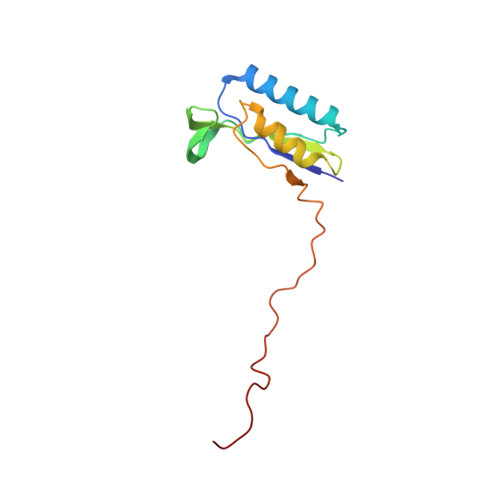
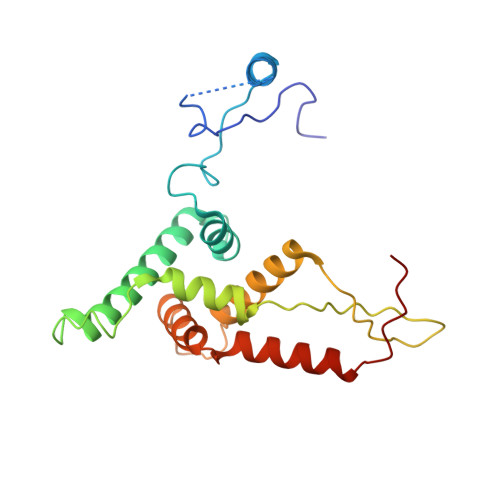
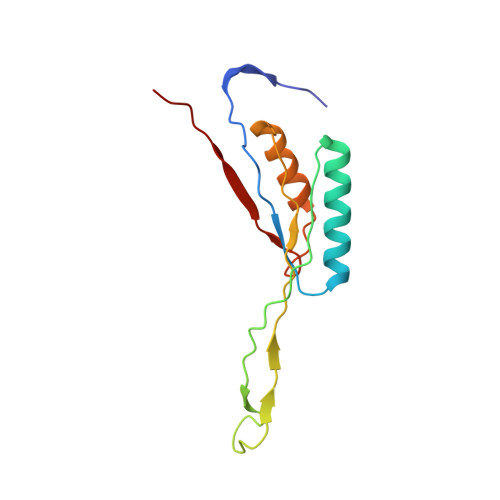
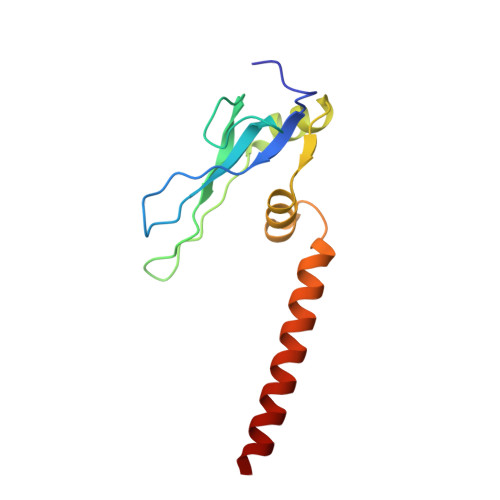
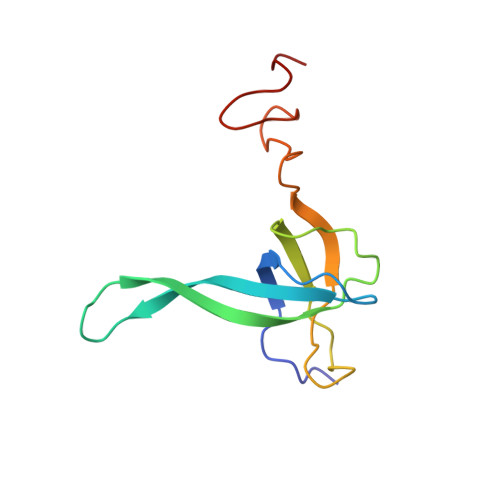
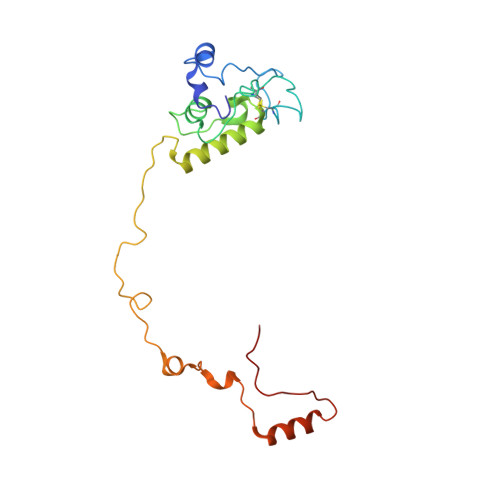
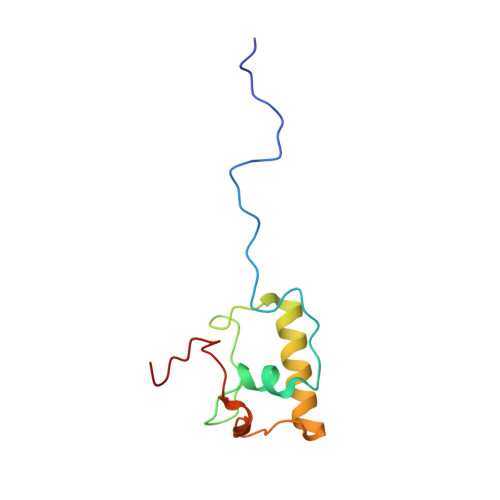
Structural insights into unique features of the human mitochondrial ribosome recycling.
Koripella, R.K., Sharma, M.R., Risteff, P., Keshavan, P., Agrawal, R.K.(2019) Proc Natl Acad Sci U S A 116: 8283-8288
- PubMed: 30962385
- DOI: https://doi.org/10.1073/pnas.1815675116
- Primary Citation of Related Structures:
6NU2, 6NU3 - PubMed Abstract:
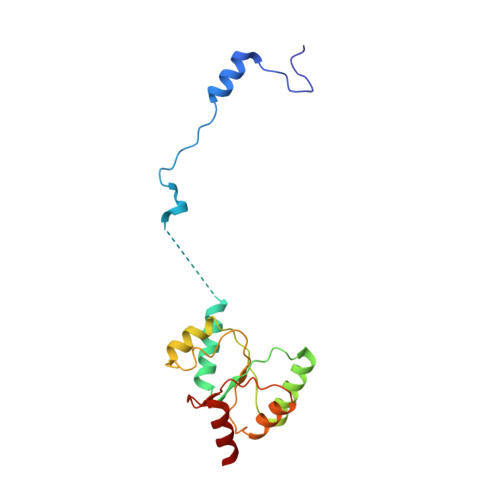
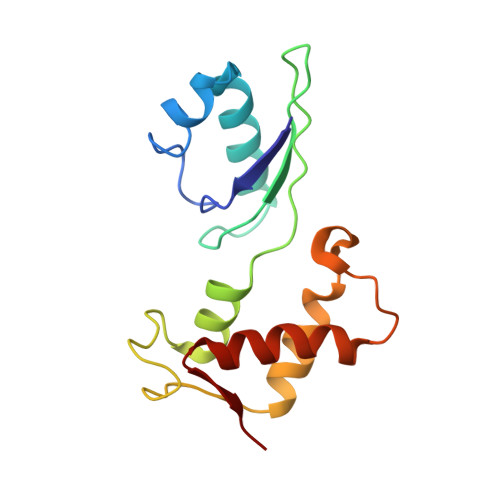
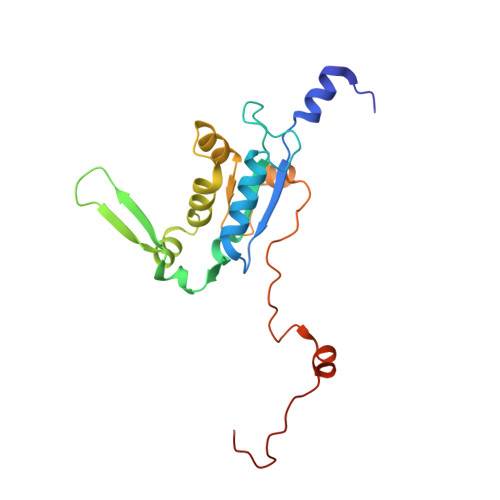
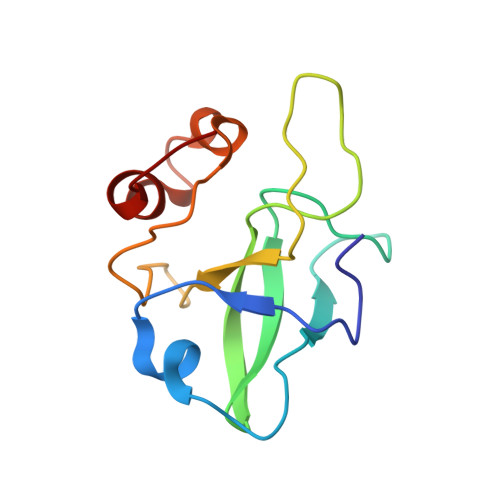
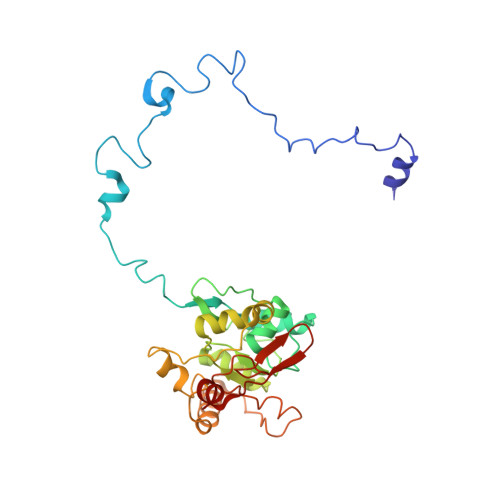
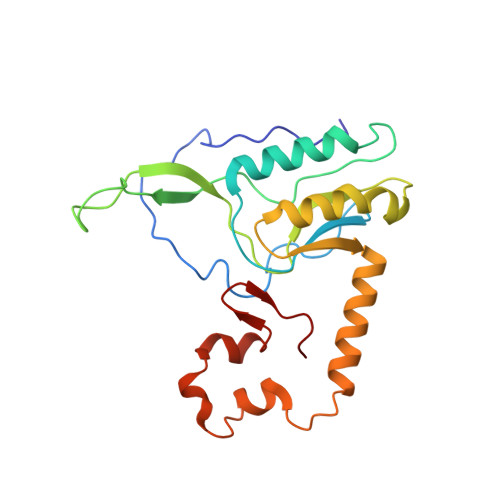
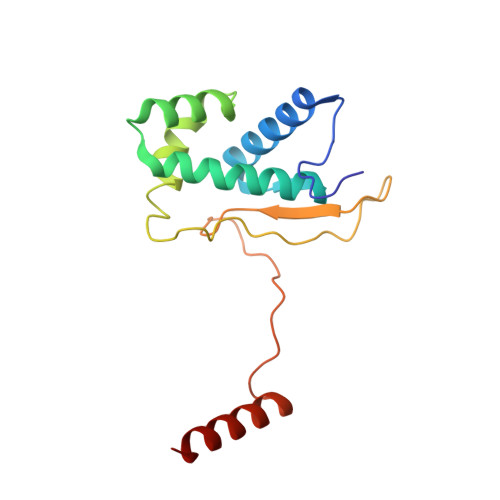
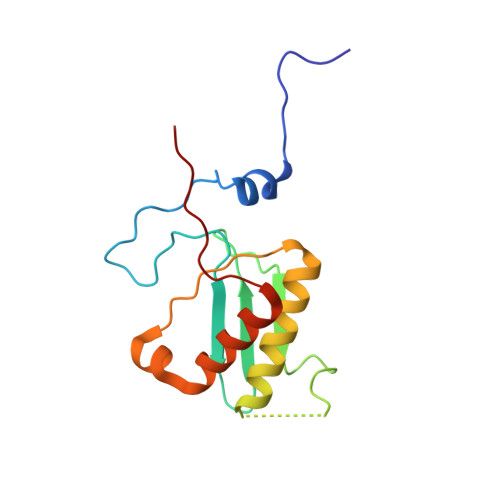
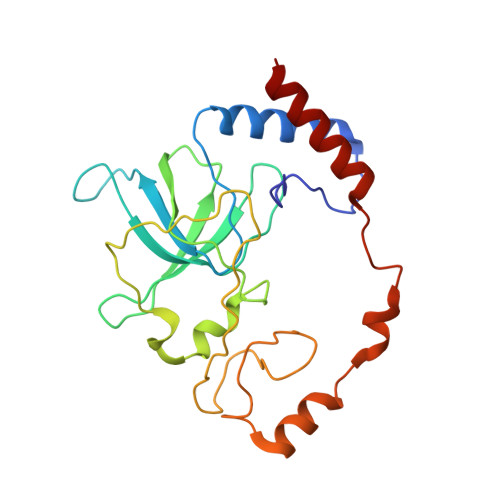
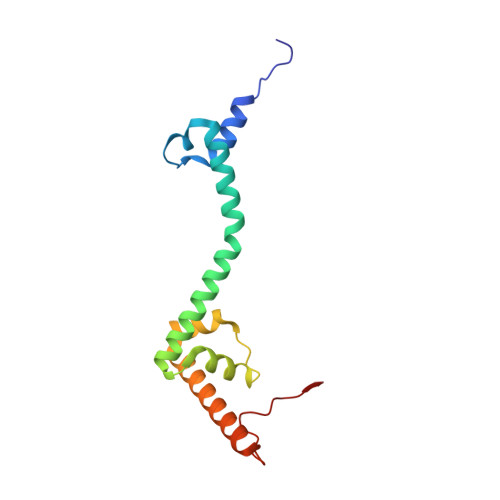
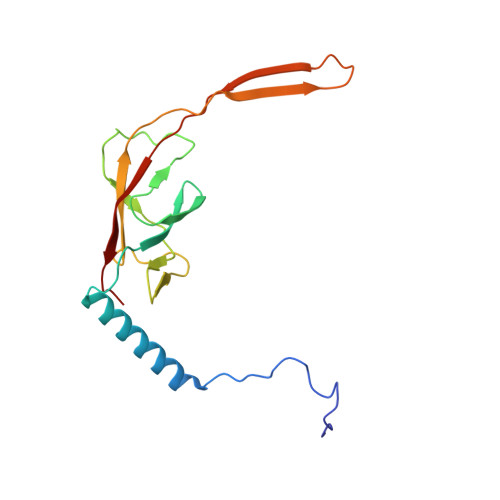
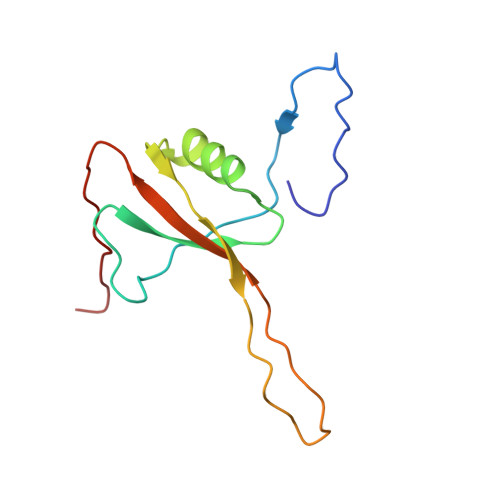
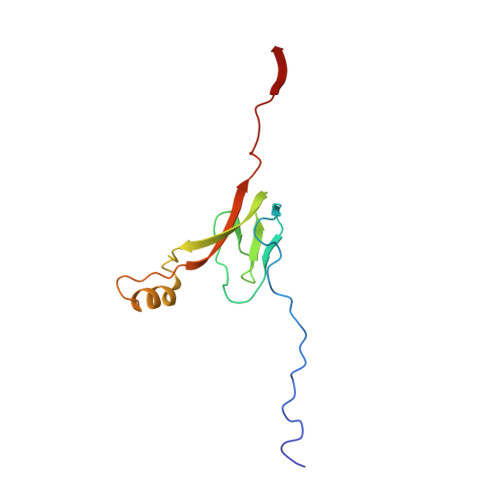
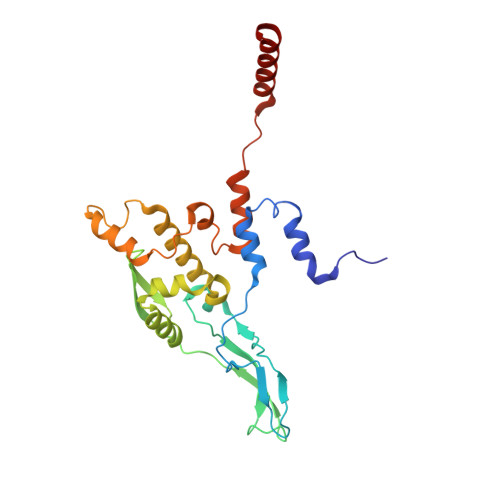
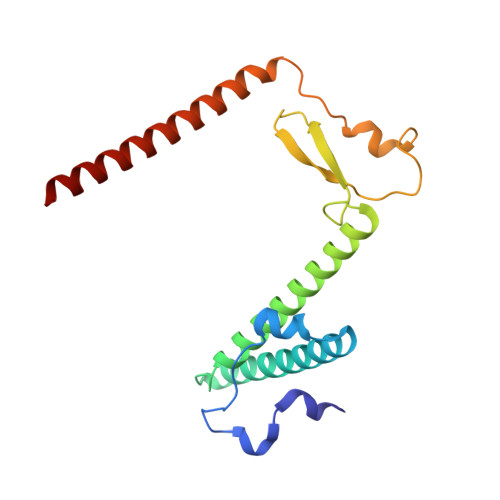
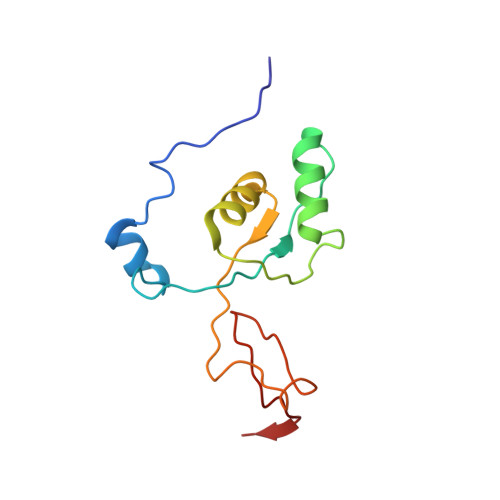
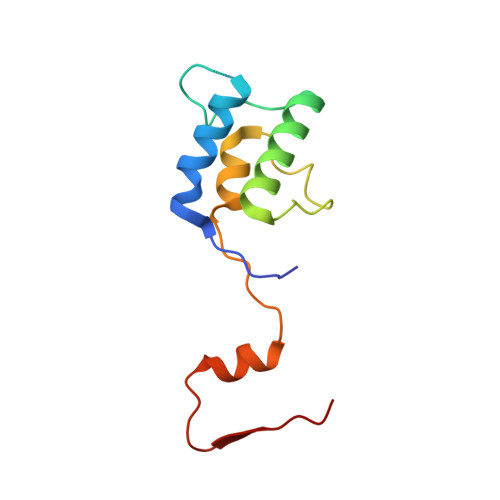
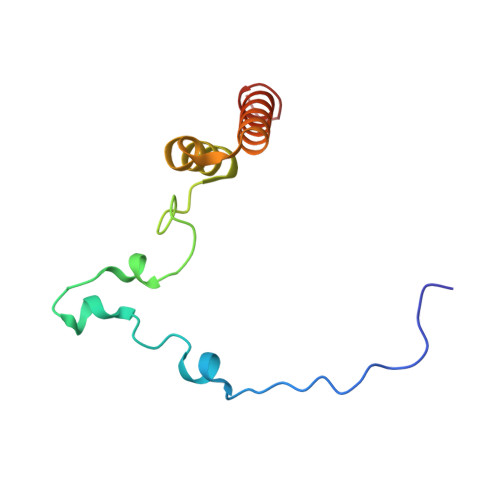
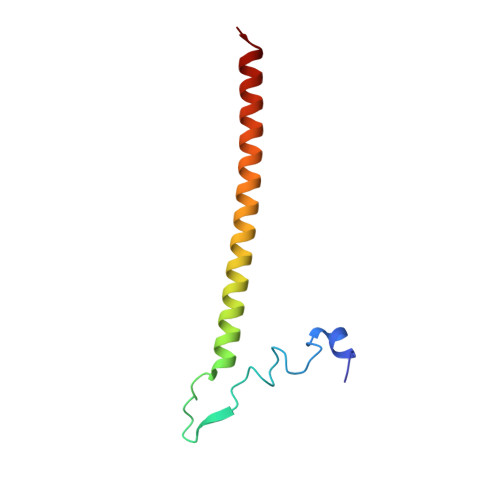
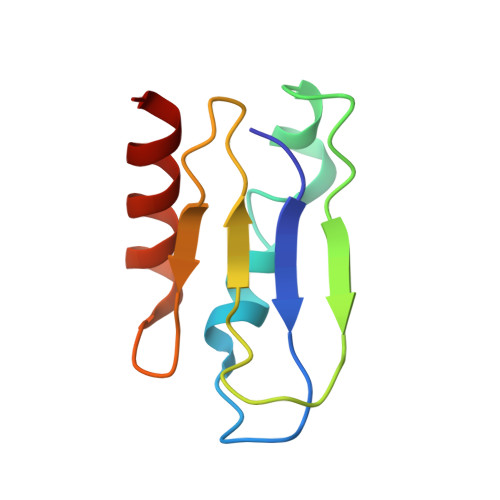
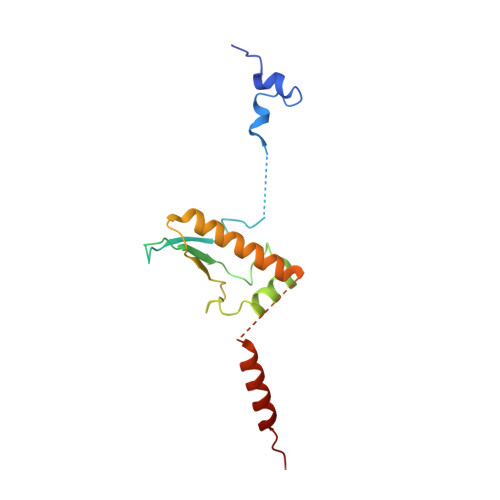
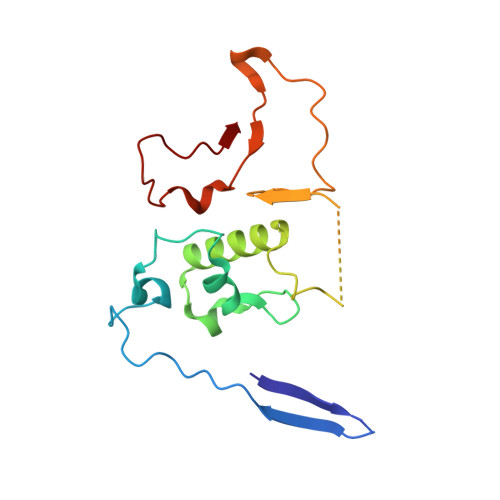
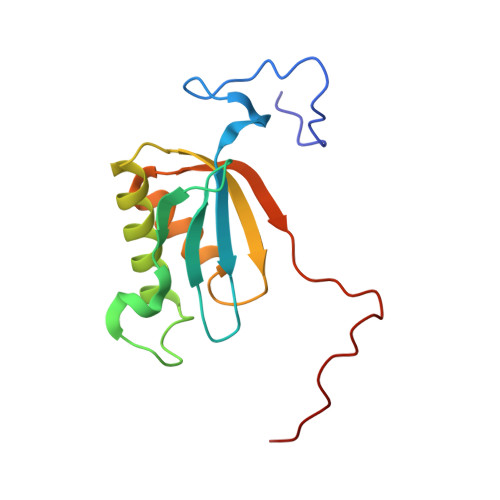
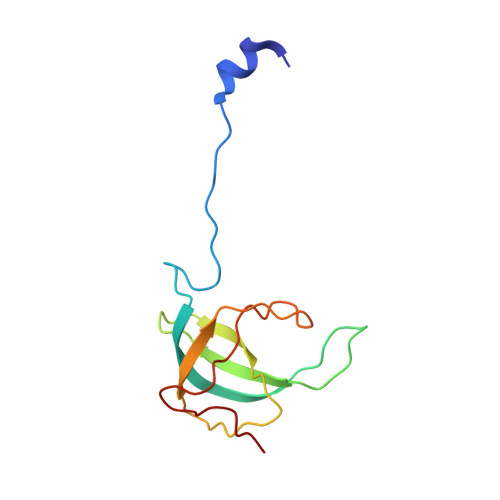
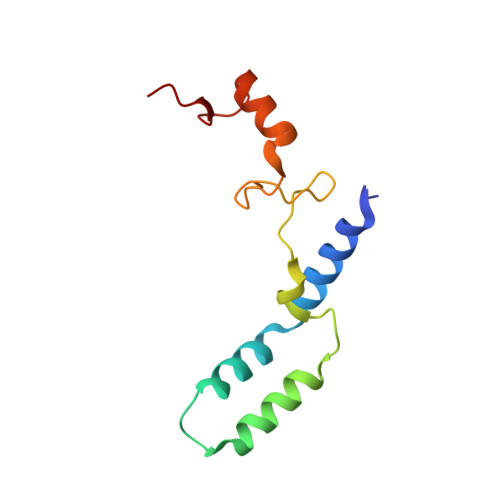
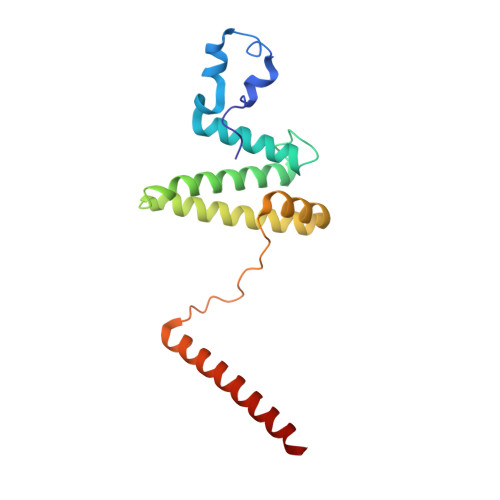
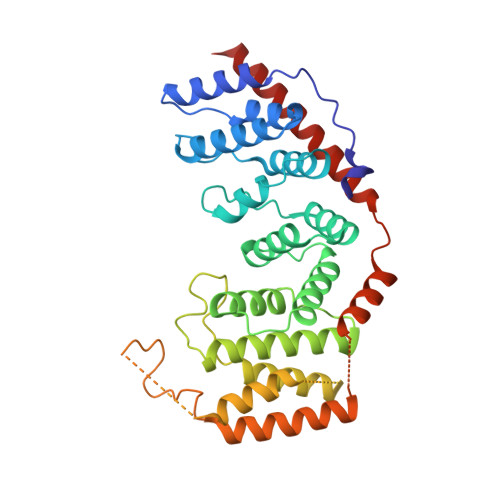
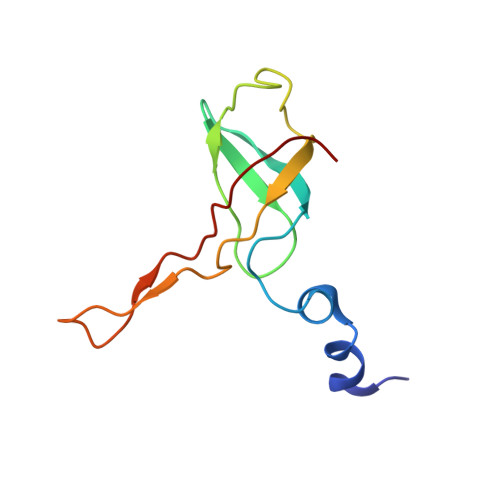
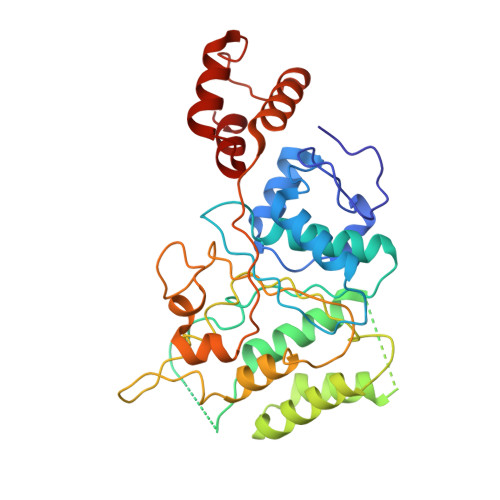
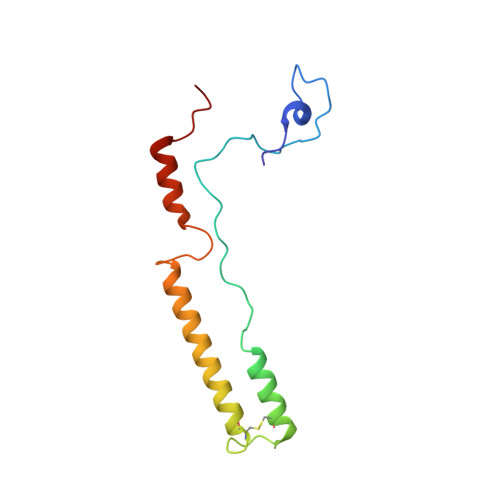
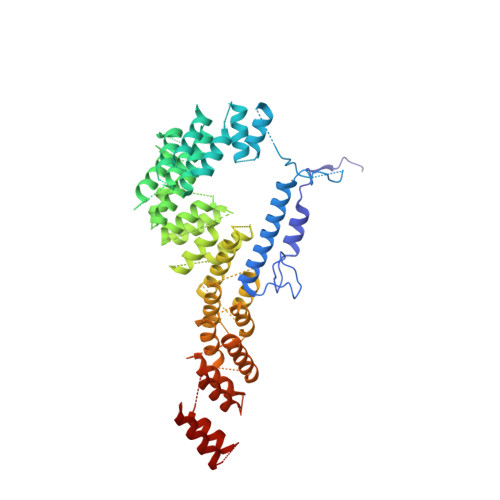
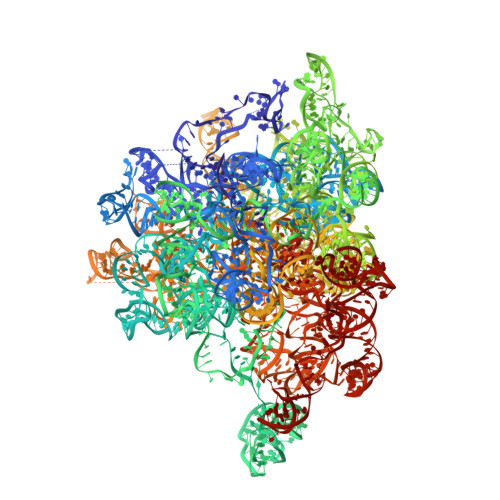
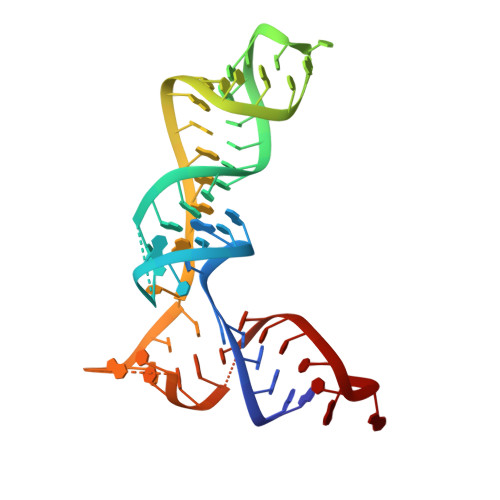
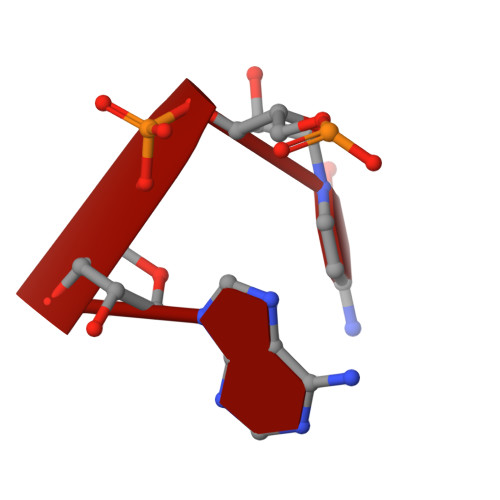
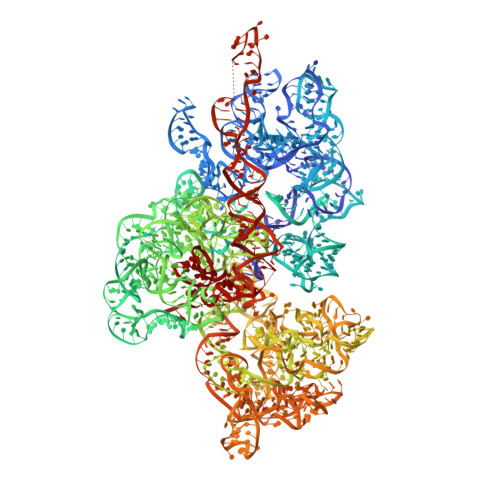
Mammalian mitochondrial ribosomes (mitoribosomes) are responsible for synthesizing proteins that are essential for oxidative phosphorylation (ATP generation). Despite their common ancestry with bacteria, the composition and structure of the human mitoribosome and its translational factors are significantly different from those of their bacterial counterparts. The mammalian mitoribosome recycling factor (RRF mt ) carries a mito-specific N terminus extension (NTE), which is necessary for the function of RRF mt Here we present a 3.9-Å resolution cryo-electron microscopic (cryo-EM) structure of the human 55S mitoribosome-RRF mt complex, which reveals α-helix and loop structures for the NTE that makes multiple mito-specific interactions with functionally critical regions of the mitoribosome. These include ribosomal RNA segments that constitute the peptidyl transferase center (PTC) and those that connect PTC with the GTPase-associated center and with mitoribosomal proteins L16 and L27. Our structure reveals the presence of a tRNA in the pe/E position and a rotation of the small mitoribosomal subunit on RRF mt binding. In addition, we observe an interaction between the pe/E tRNA and a mito-specific protein, mL64. These findings help understand the unique features of mitoribosome recycling.
- Division of Translational Medicine, Wadsworth Center, New York State Department of Health, Albany, NY 12201-0509.
Organizational Affiliation: