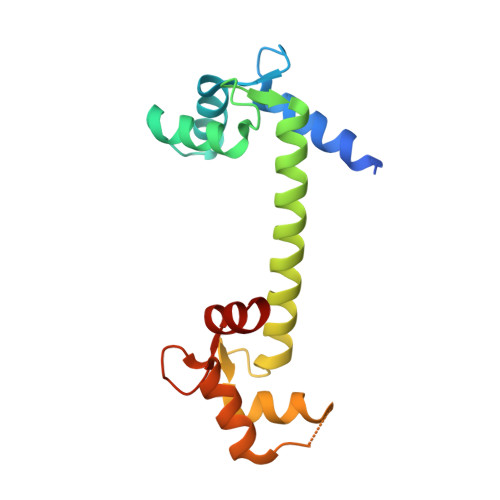
Crystal structures of Ca2+-calmodulin bound to NaVC-terminal regions suggest role for EF-hand domain in binding and inactivation.
Gardill, B.R., Rivera-Acevedo, R.E., Tung, C.C., Van Petegem, F.(2019) Proc Natl Acad Sci U S A 116: 10763-10772
- PubMed: 31072926
- DOI: https://doi.org/10.1073/pnas.1818618116
- Primary Citation of Related Structures:
6MUD, 6MUE - PubMed Abstract:
Voltage-gated sodium (Na V ) and calcium channels (Ca V ) form targets for calmodulin (CaM), which affects channel inactivation properties. A major interaction site for CaM resides in the C-terminal (CT) region, consisting of an IQ domain downstream of an EF-hand domain. We present a crystal structure of fully Ca 2+ -occupied CaM, bound to the CT of Na V 1.5. The structure shows that the C-terminal lobe binds to a site ∼90° rotated relative to a previous site reported for an apoCaM complex with the Na V 1.5 CT and for ternary complexes containing fibroblast growth factor homologous factors (FHF). We show that the binding of FHFs forces the EF-hand domain in a conformation that does not allow binding of the Ca 2+ -occupied C-lobe of CaM. These observations highlight the central role of the EF-hand domain in modulating the binding mode of CaM. The binding sites for Ca 2+ -free and Ca 2+ -occupied CaM contain targets for mutations linked to long-QT syndrome, a type of inherited arrhythmia. The related Na V 1.4 channel has been shown to undergo Ca 2+ -dependent inactivation (CDI) akin to Ca V s. We present a crystal structure of Ca 2+ /CaM bound to the Na V 1.4 IQ domain, which shows a binding mode that would clash with the EF-hand domain. We postulate the relative reorientation of the EF-hand domain and the IQ domain as a possible conformational switch that underlies CDI.
- Department of Biochemistry and Molecular Biology, Life Sciences Institute, The University of British Columbia, Vancouver, BC, Canada V6T 1Z3.
Organizational Affiliation: