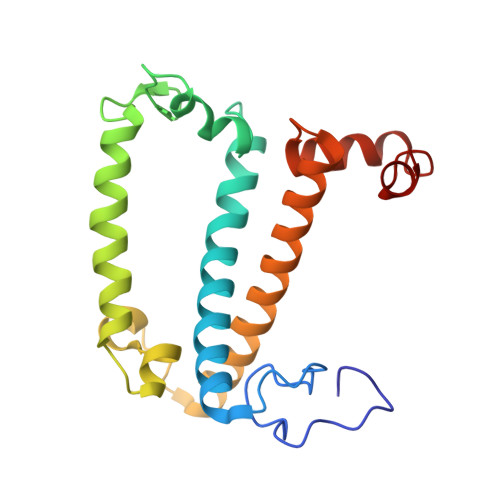
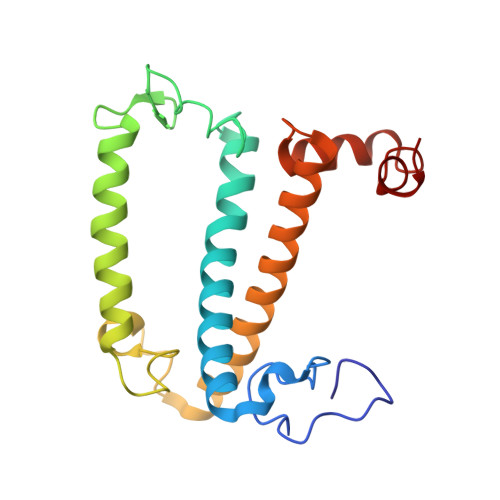
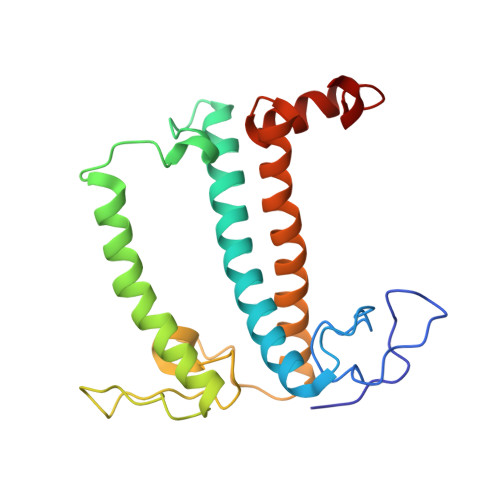
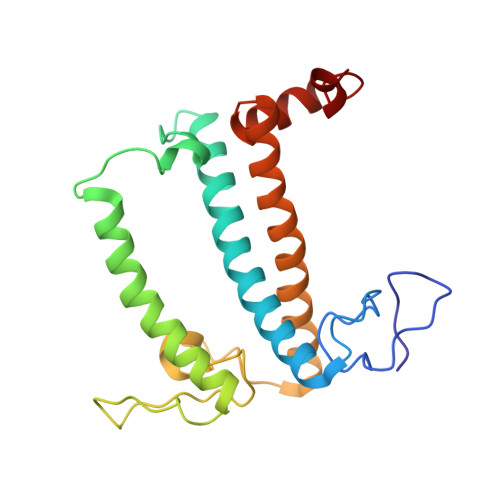
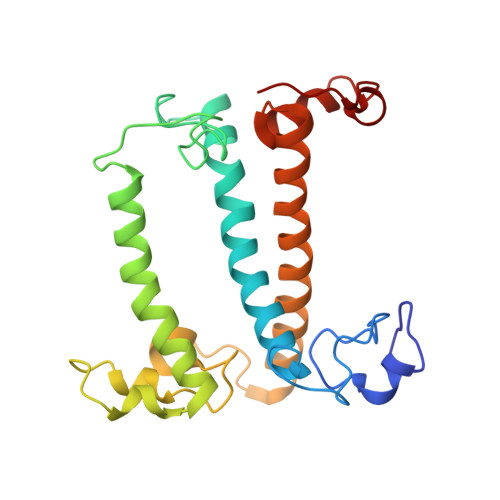
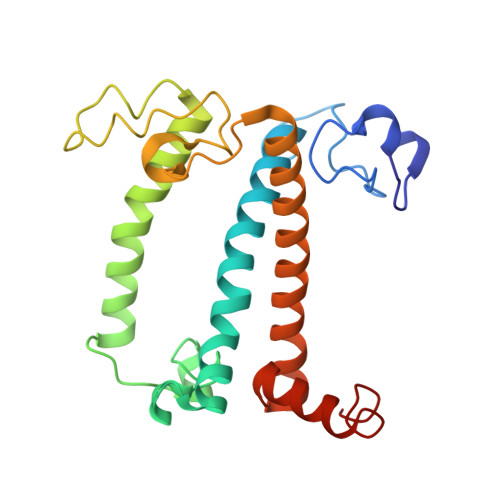
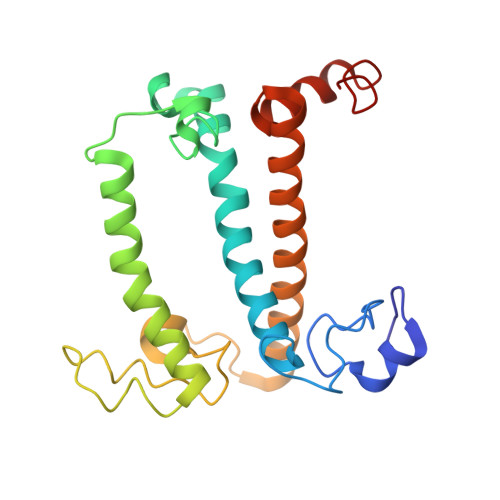
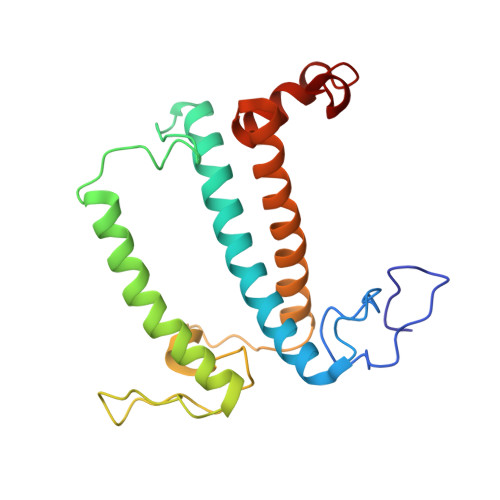
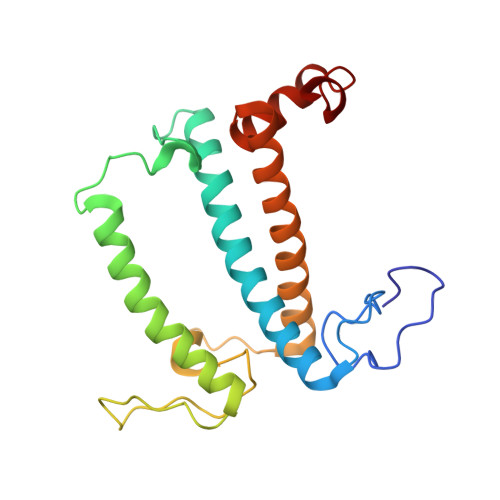
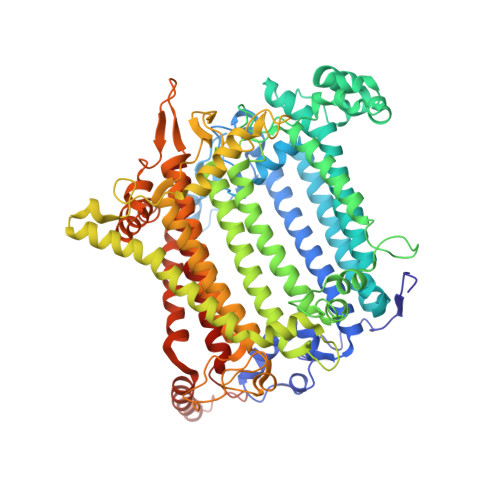
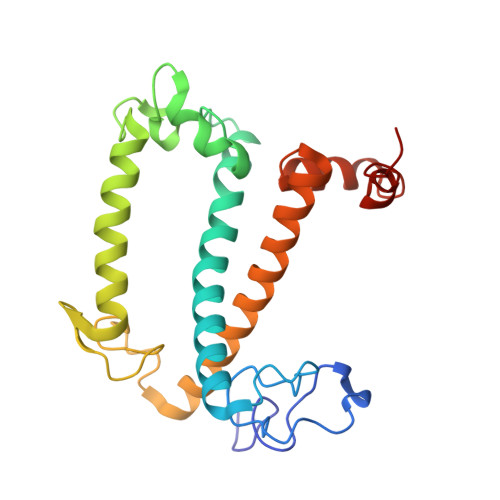
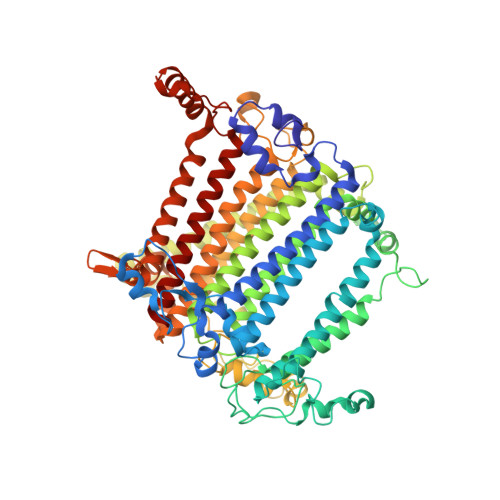
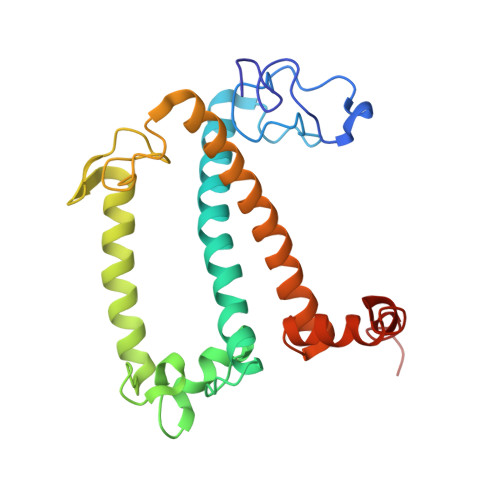
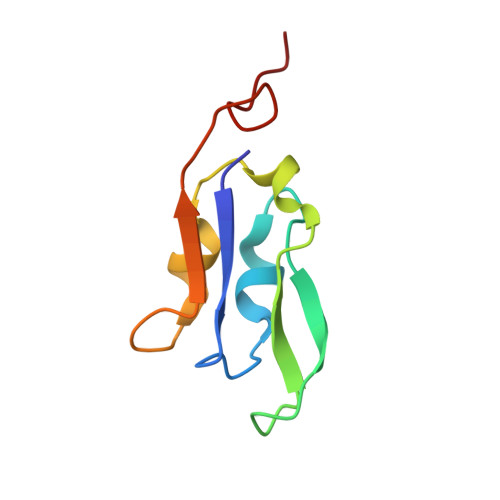
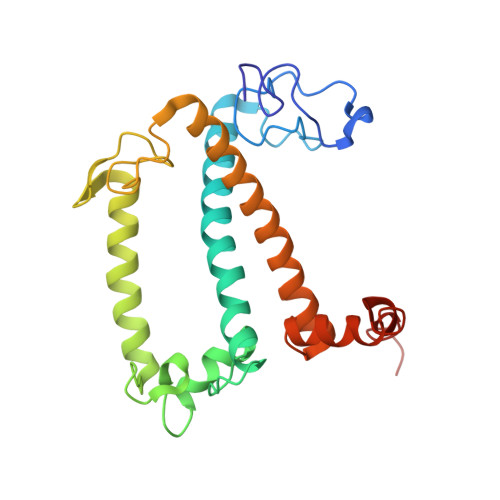
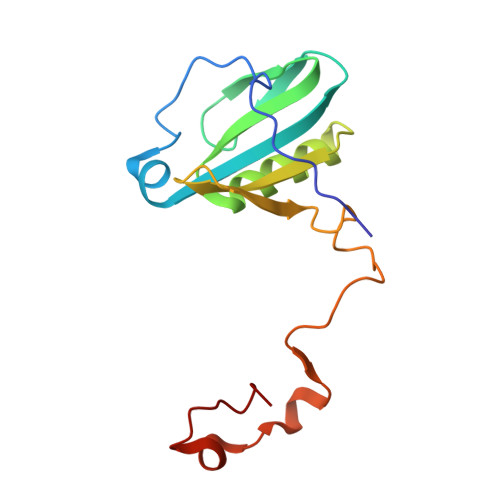
A unique supramolecular organization of photosystem I in the moss Physcomitrella patens.
Iwai, M., Grob, P., Iavarone, A.T., Nogales, E., Niyogi, K.K.(2018) Nat Plants 4: 904-909
- PubMed: 30374090
- DOI: https://doi.org/10.1038/s41477-018-0271-1
- Primary Citation of Related Structures:
6MEM - PubMed Abstract:
The photosynthesis machinery in chloroplast thylakoid membranes is comprised of multiple protein complexes and supercomplexes 1,2 . Here, we show a novel supramolecular organization of photosystem I (PSI) in the moss Physcomitrella patens by single-particle cryo-electron microscopy. The moss-specific light-harvesting complex (LHC) protein Lhcb9 is involved in this PSI supercomplex, which has been shown to have a molecular density similar to that of the green alga Chlamydomonas reinhardtii 3 . Our results show that the structural organization is unexpectedly different-two rows of the LHCI belt exist as in C. reinhardtii 4 , but the outer one is shifted toward the PsaK side. Furthermore, one trimeric LHC protein and one monomeric LHC protein position alongside PsaL/K, filling the gap between these subunits and the outer LHCI belt. We provide evidence showing that Lhcb9 is a key factor, acting as a linkage between the PSI core and the outer LHCI belt to form the unique supramolecular organization of the PSI supercomplex in P. patens.
- Department of Plant and Microbial Biology, University of California, Berkeley, CA, USA. miwai@berkeley.edu.
Organizational Affiliation: