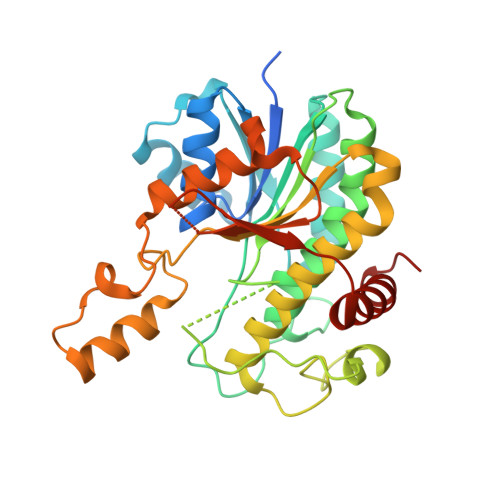
Crystal structures of cyanobacterial light-dependent protochlorophyllide oxidoreductase.
Dong, C.S., Zhang, W.L., Wang, Q., Li, Y.S., Wang, X., Zhang, M., Liu, L.(2020) Proc Natl Acad Sci U S A 117: 8455-8461
- PubMed: 32234783
- DOI: https://doi.org/10.1073/pnas.1920244117
- Primary Citation of Related Structures:
6L1G, 6L1H - PubMed Abstract:
The reduction of protochlorophyllide (Pchlide) to chlorophyllide (Chlide) is the penultimate step of chlorophyll biosynthesis. In oxygenic photosynthetic bacteria, algae, and plants, this reaction can be catalyzed by the light-dependent Pchlide oxidoreductase (LPOR), a member of the short-chain dehydrogenase superfamily sharing a conserved Rossmann fold for NAD(P)H binding and the catalytic activity. Whereas modeling and simulation approaches have been used to study the catalytic mechanism of this light-driven reaction, key details of the LPOR structure remain unclear. We determined the crystal structures of LPOR from two cyanobacteria, Synechocystis sp. PCC 6803 and Thermosynechococcus elongatus Structural analysis defines the LPOR core fold, outlines the LPOR-NADPH interaction network, identifies the residues forming the substrate cavity and the proton-relay path, and reveals the role of the LPOR-specific loop. These findings provide a basis for understanding the structure-function relationships of the light-driven Pchlide reduction.
- School of Life Sciences, Anhui University, 230601 Hefei, Anhui, China.
Organizational Affiliation: