Crystal structure of the N-terminal domain single mutant (D119A) of the human mitochondrial calcium uniporter fused with T4 lysozyme
Lee, Y., Park, J.S., Kang, J.Y., Eom, S.H.(2020) Sci Rep
Experimental Data Snapshot
Starting Models: experimental
View more details
wwPDB Validation 3D Report Full Report
(2020) Sci Rep
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Endolysin,Calcium uniporter protein, mitochondrial | 261 | Tequatrovirus T4, Homo sapiens This entity is chimeric | Mutation(s): 4 Gene Names: e, T4Tp126, MCU, C10orf42, CCDC109A EC: 3.2.1.17 | 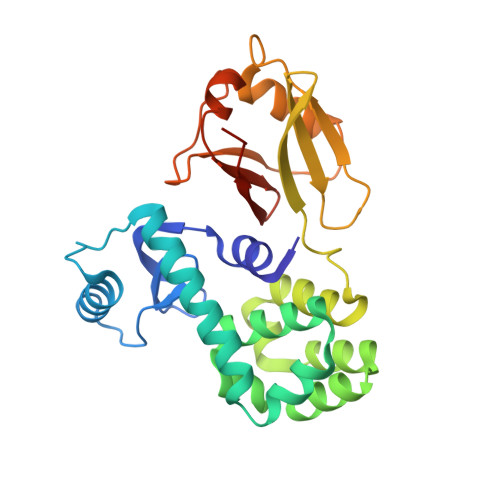 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for D9IEF7 (Enterobacteria phage T4) Explore D9IEF7 Go to UniProtKB: D9IEF7 | |||||
Find proteins for Q8NE86 (Homo sapiens) Explore Q8NE86 Go to UniProtKB: Q8NE86 | |||||
PHAROS: Q8NE86 GTEx: ENSG00000156026 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Groups | D9IEF7Q8NE86 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| SO4 Query on SO4 | B [auth A], C [auth A], D [auth A], E [auth A] | SULFATE ION O4 S QAOWNCQODCNURD-UHFFFAOYSA-L |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 97.899 | α = 90 |
| b = 97.899 | β = 90 |
| c = 61.619 | γ = 120 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| HKL-2000 | data scaling |
| Coot | model building |
| PHASER | phasing |
| HKL-2000 | data reduction |