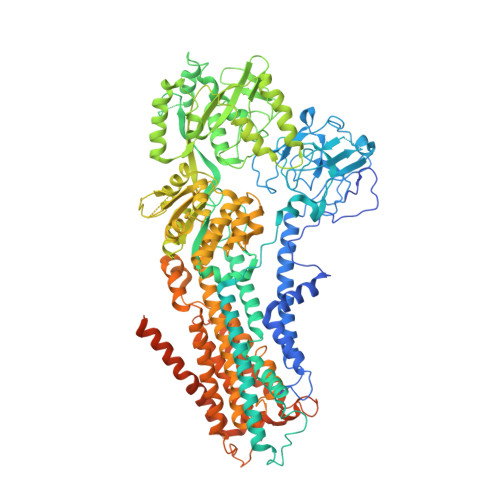
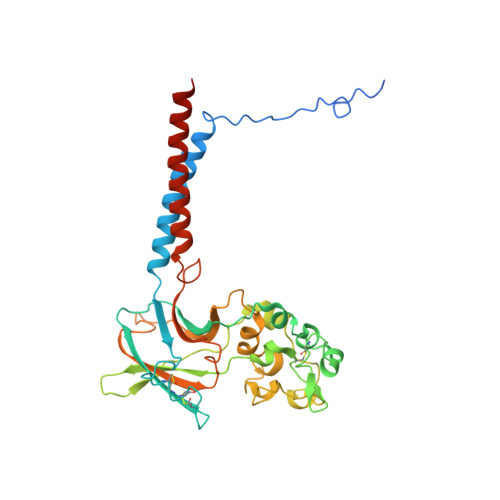
Cryo-EM structures capture the transport cycle of the P4-ATPase flippase.
Hiraizumi, M., Yamashita, K., Nishizawa, T., Nureki, O.(2019) Science 365: 1149-1155
- PubMed: 31416931
- DOI: https://doi.org/10.1126/science.aay3353
- Primary Citation of Related Structures:
6K7G, 6K7H, 6K7I, 6K7J, 6K7K, 6K7L, 6K7M, 6K7N - PubMed Abstract:
In eukaryotic membranes, type IV P-type adenosine triphosphatases (P4-ATPases) mediate the translocation of phospholipids from the outer to the inner leaflet and maintain lipid asymmetry, which is critical for membrane trafficking and signaling pathways. Here, we report the cryo-electron microscopy structures of six distinct intermediates of the human ATP8A1-CDC50a heterocomplex at resolutions of 2.6 to 3.3 angstroms, elucidating the lipid translocation cycle of this P4-ATPase. ATP-dependent phosphorylation induces a large rotational movement of the actuator domain around the phosphorylation site in the phosphorylation domain, accompanied by lateral shifts of the first and second transmembrane helices, thereby allowing phosphatidylserine binding. The phospholipid head group passes through the hydrophilic cleft, while the acyl chain is exposed toward the lipid environment. These findings advance our understanding of the flippase mechanism and the disease-associated mutants of P4-ATPases.
- Department of Biological Sciences, Graduate School of Science, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, 113-0033, Japan.
Organizational Affiliation: