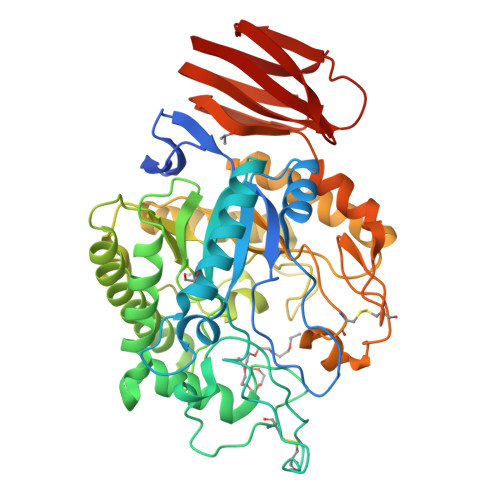
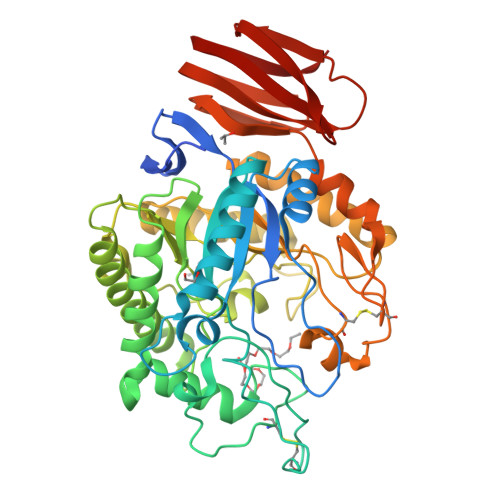
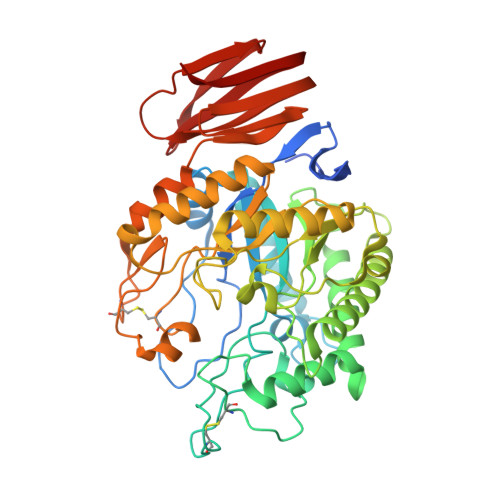
Structure of an endogalactosylceramidase from Rhodococcus hoagii 103S reveals the molecular basis of its substrate specificity.
Chen, L., Chang, Q., Yan, Q., Yang, G., Zhang, Y., Feng, Y.(2019) J Struct Biol 208: 107393-107393
- PubMed: 31557527
- DOI: https://doi.org/10.1016/j.jsb.2019.09.010
- Primary Citation of Related Structures:
6JYZ - PubMed Abstract:
Endoglycoceramidases (EGCs) are family 5 glycoside hydrolases that catalyze hydrolysis of the glycosidic linkages between the oligosaccharide and ceramide moieties of glycosphingolipids. Three orthologs of EGCs, each with distinct substrate specificity, have been identified to date, including EGC-I, EGC-II, and EGALC. Although the structures of EGC-I and EGC-II have been reported, the substrate preference mechanism of EGC enzymes remains unclear. Here, we determined the crystal structure of EGALC from Rhodococcus hoagii 103S at a resolution of 1.20 Å. Distinct from EGC-I and EGC-II, which both have a tunnel-like substrate binding site, the structure of EGALC accommodates substrates in a long groove. Further, the oligosaccharide-binding region of groove could be divided into two small pockets that separately bind to the Gal1 and to the Gal3/Gla3 present in 6-gala series substrates. Structural and sequence comparisons of EGC enzymes revealed that the conformation and length of their Nβ8-Lα1 regions are crucial in determining the architectures of their specific substrate binding sites. Importantly, molecular docking analyses indicate that the substrate specificity of each EGC is mainly derived from the complementarity of its active site groove/tunnel with substrates adopting particular conformations. Our study provide insights for understanding the catalytic mechanism of EGALC, which will help protein engineering for improving the substrate preference and catalytic efficiency of EGC enzymes toward important glycosphingolipid substrates.
Organizational Affiliation:
State Key Laboratory of Microbial Metabolism, School of Life Sciences and Biotechnology, Shanghai Jiao Tong University, Shanghai 200240, China; Research Center for Computer-Aided Drug Discovery, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen 518055, China.