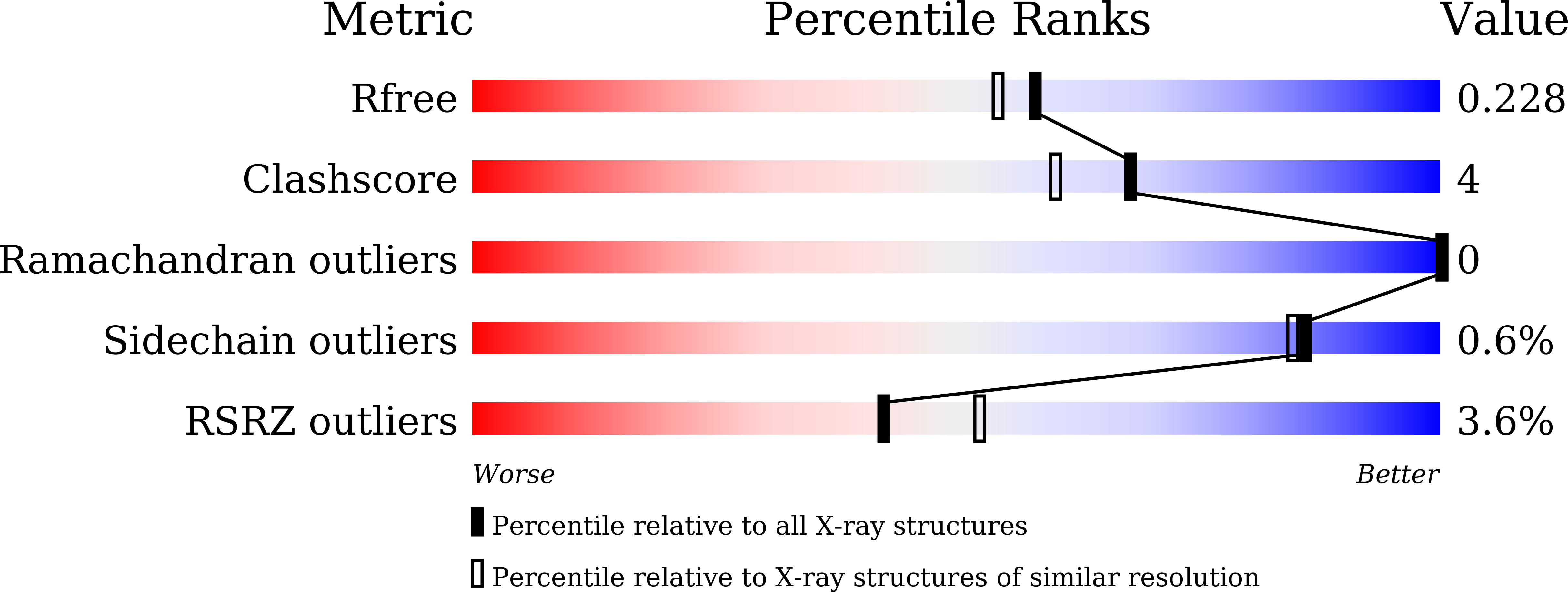
BubR1 phosphorylates CENP-E as a switch enabling the transition from lateral association to end-on capture of spindle microtubules.
Huang, Y., Lin, L., Liu, X., Ye, S., Yao, P.Y., Wang, W., Yang, F., Gao, X., Li, J., Zhang, Y., Zhang, J., Yang, Z., Liu, X., Yang, Z., Zang, J., Teng, M., Wang, Z., Ruan, K., Ding, X., Li, L., Cleveland, D.W., Zhang, R., Yao, X.(2019) Cell Res 29: 562-578
- PubMed: 31201382
- DOI: https://doi.org/10.1038/s41422-019-0178-z
- Primary Citation of Related Structures:
6JKK, 6JKM - PubMed Abstract:
Error-free mitosis depends on accurate chromosome attachment to spindle microtubules, powered congression of those chromosomes, their segregation in anaphase, and assembly of a spindle midzone at mitotic exit. The centromere-associated kinesin motor CENP-E, whose binding partner is BubR1, has been implicated in congression of misaligned chromosomes and the transition from lateral kinetochore-microtubule association to end-on capture. Although previously proposed to be a pseudokinase, here we report the structure of the kinase domain of Drosophila melanogaster BubR1, revealing its folding into a conformation predicted to be catalytically active. BubR1 is shown to be a bona fide kinase whose phosphorylation of CENP-E switches it from a laterally attached microtubule motor to a plus-end microtubule tip tracker. Computational modeling is used to identify bubristatin as a selective BubR1 kinase antagonist that targets the αN1 helix of N-terminal extension and αC helix of the BubR1 kinase domain. Inhibition of CENP-E phosphorylation is shown to prevent proper microtubule capture at kinetochores and, surprisingly, proper assembly of the central spindle at mitotic exit. Thus, BubR1-mediated CENP-E phosphorylation produces a temporal switch that enables transition from lateral to end-on microtubule capture and organization of microtubules into stable midzone arrays.
Organizational Affiliation:
Anhui Key Laboratory for Chemical Biology & MOE Key Laboratory for Cellular Dynamics, CAS Center for Excellence in Molecular Cell Science, School of Life Sciences, Hefei National Laboratory for Physical Sciences at the Mciroscale, University of Science & Technology of China, Hefei, Anhui, 230026, China.