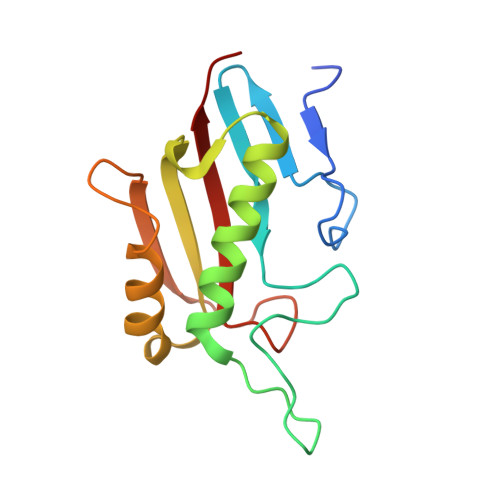
A Unique Homo-Hexameric Structure of 2-Aminomuconate Deaminase in the BacteriumPseudomonas species AP-3.
Chen, Y., Chen, Y., Jiang, H., Lu, D., Hu, T., Bi, G., Ran, Y., Yu, B., Dong, H., Su, D.(2019) Front Microbiol 10: 2079-2079
- PubMed: 31555255
- DOI: https://doi.org/10.3389/fmicb.2019.02079
- Primary Citation of Related Structures:
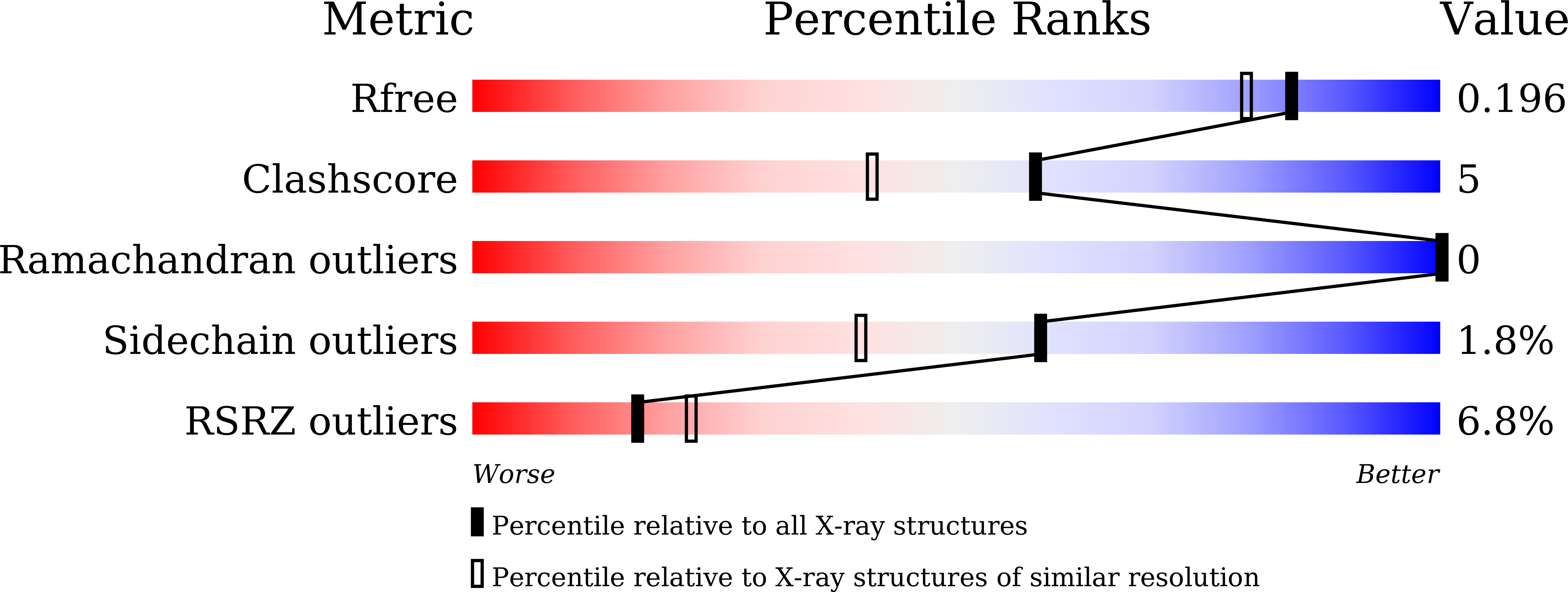
6IZH - PubMed Abstract:
The bacterium Pseudomonas species sp. AP-3 is one of several microorganisms that are capable of using 2-aminophenol as its sole source of carbon, nitrogen and energy. Several 2-aminophenol-metabolizing enzymes have pivotal roles in the biodegradation of aniline and its derivatives as environmental pollutants in Pseudomonas . The bacterium Pseudomonas sp. AP-3 recruits a unique 2-aminomuconate deaminase (AmnE) to hydrolyze 2-aminomuconate to 4-oxalocrotonate, and releases ammonia in the modified meta-cleavage pathway by forming various compounds-including acetaldehyde, pyruvic acid, acetyl-CoA, and succinate-that may enter the Krebs cycle. AmnE also belongs to the YjgF/YER057c/UK114 family (also known as the Rid family), which is conserved in all domains of life and prefers structurally homotrimeric forms with diverse functional purposes. To study the mechanism of the modified meta-cleavage pathway in Pseudomonas sp. AP-3 , we determined the first crystal structure of AmnE from Pseudomonas sp. AP-3 at 1.75 Å. AmnE forms a unique homohexamer instead of a trimer which is normally adopted by the members of YjgF/YER057c/UK114 family. Based on the structure of the AmnE hexamer, we observed a hydrophobic base composed of six Lp3 loops (residues 122-131) in each of the AmnE protomers that have pivotal roles in the assembly of the hexamer. Eighteen hydrogen bonds formed by the residues Met 96 , Pro 126 , and Arg 56 , which surround the hydrophobic base, allowed the combination of the two trimers into a stable hexamer. The single mutant of AmnE R56A lost the ability to maintain the hexameric conformation, and revealed that the hydrogen bonds between residues Arg 56 and Met 96 have pivotal roles in the AmnE hexameric assembly.
Organizational Affiliation:
State Key Laboratory of Biotherapy and Cancer Center, West China Hospital, Sichuan University and Collaborative Innovation Center of Biotherapy, Chengdu, China.