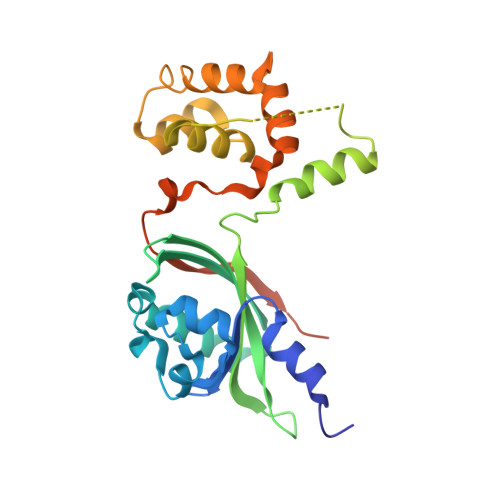
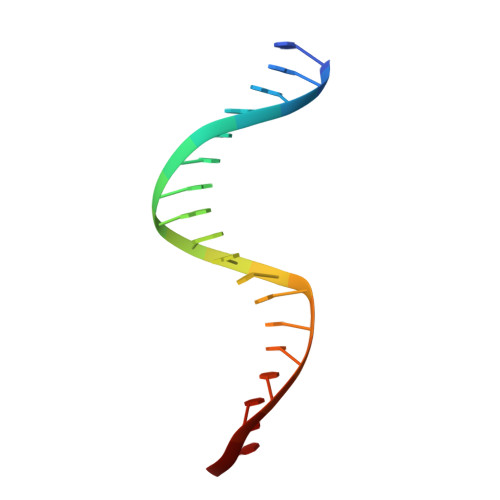
Crystal structure of theVibrio choleraeVqmA-ligand-DNA complex provides insight into ligand-binding mechanisms relevant for drug design.
Wu, H., Li, M., Guo, H., Zhou, H., Li, B., Xu, Q., Xu, C., Yu, F., He, J.(2019) J Biological Chem 294: 2580-2592
- PubMed: 30610119
- DOI: https://doi.org/10.1074/jbc.RA118.006082
- Primary Citation of Related Structures:
6IDE - PubMed Abstract:
VqmA is a highly conserved transcriptional regulator of the quorum-sensing system of Vibrio cholerae , a major human pathogen that continues to imperil human health. VqmA represses biofilm formation and plays an important role in V. cholerae pathogenicity in the human host. Although VqmA's biological function is well understood, the molecular mechanisms by which its specific ligand (and effector), 3,5-dimethylpyrazine-2-ol (DPO), controls transcription of the target gene, vqmR , remain obscure. To elucidate the molecular mechanism of DPO binding, we used structural analyses and biochemical assays to study the V. cholerae VqmA-DPO-DNA complex. These analyses revealed that VqmA contains an N-terminal homodimer domain (PAS) and a C-terminal DNA-binding domain (DBD). We observed that VqmA directly binds to a DPO molecule via a compact hydrophobic pocket, consisting of a six-stranded antiparallel β-sheet and several α-helices. We also found that the VqmA dimer interacts with the quasi-palindromic sequence of the vqmR promoter through its DBD. The results of the biochemical studies indicated that a water atom and VqmA residues Phe-67 and Lys-101 play a key role in effector recognition, which is also assisted by Tyr-36 and Phe-99. This is the first molecular level view of the VqmA dimer bound to DPO and DNA. The structure-function analyses presented here improve our understanding of the complex mechanisms in the transcriptional regulation of VqmA in Vibrio spp. and may inform the design of drugs to manage V. cholerae infections.
- From the Shanghai Institute of Applied Physics, Chinese Academy of Sciences, Shanghai 201800 and.
Organizational Affiliation: