GFP8 - a stabilized variant of cycle-3 GFP
Petek, M., Rangel Pereira, M.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Green fluorescent protein | 249 | Aequorea victoria | Mutation(s): 13 Gene Names: gfp | 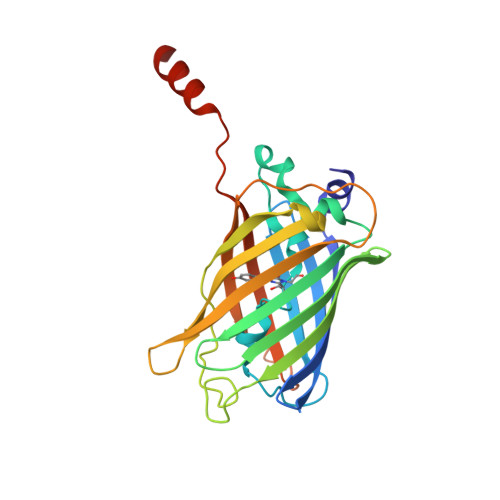 | |
UniProt | |||||
Find proteins for P42212 (Aequorea victoria) Explore P42212 Go to UniProtKB: P42212 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P42212 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| CRO Query on CRO | A | L-PEPTIDE LINKING | C15 H17 N3 O5 |  | THR, TYR, GLY |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 52.16 | α = 90 |
| b = 63.37 | β = 90 |
| c = 69.21 | γ = 90 |
| Software Name | Purpose |
|---|---|
| AMoRE | phasing |
| PHENIX | refinement |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Biotechnology and Biological Sciences Research Council | United Kingdom | BB/M011194/1 |