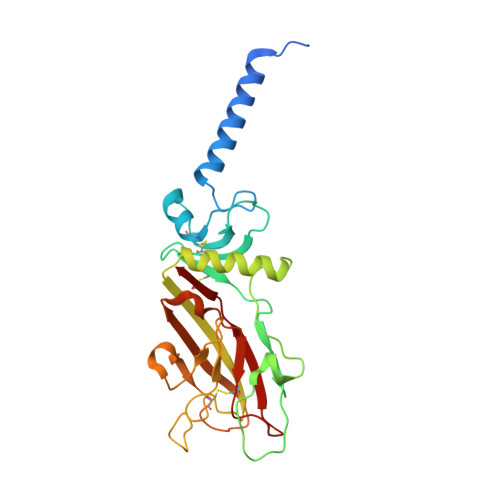
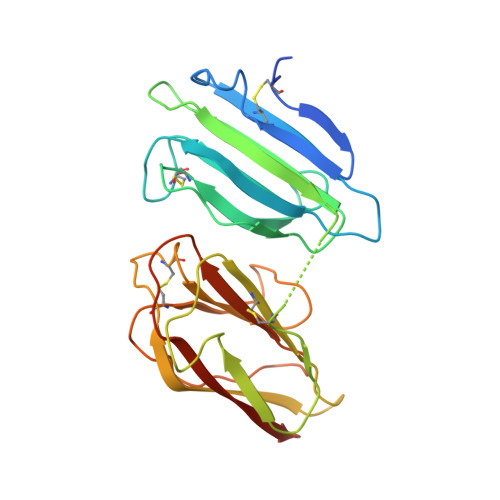
Structural Basis for the Acceleration of Procollagen Processing by Procollagen C-Proteinase Enhancer-1.
Pulido, D., Sharma, U., Vadon-Le Goff, S., Hussain, S.A., Cordes, S., Mariano, N., Bettler, E., Moali, C., Aghajari, N., Hohenester, E., Hulmes, D.J.S.(2018) Structure 26: 1384-1392.e3
- PubMed: 30078642
- DOI: https://doi.org/10.1016/j.str.2018.06.011
- Primary Citation of Related Structures:
6FZV, 6FZW - PubMed Abstract:
Procollagen C-proteinase enhancer-1 (PCPE-1) is a secreted protein that specifically accelerates proteolytic release of the C-propeptides from fibrillar procollagens, a crucial step in fibril assembly. As such, it is a potential therapeutic target to improve tissue repair and prevent fibrosis, a major cause of mortality worldwide. Here we present the crystal structure of the active CUB1CUB2 fragment of PCPE-1 bound to the C-propeptide trimer of procollagen III (CPIII). This shows that the two CUB domains bind to two different chains of CPIII and that the N-terminal region of one CPIII chain, close to the proteolytic cleavage site, lies in the cleft between CUB1 and CUB2. This suggests that enhancing activity involves unraveling of this chain from the rest of the trimer, thus facilitating the action of the proteinase involved. Support for this hypothesis comes from site-directed mutagenesis, enzyme assays, binding studies, and molecular modeling.
- Department of Life Sciences, Imperial College, London SW7 2AZ, UK.
Organizational Affiliation: