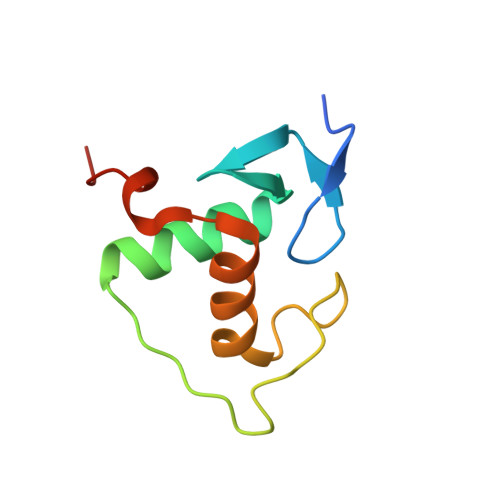
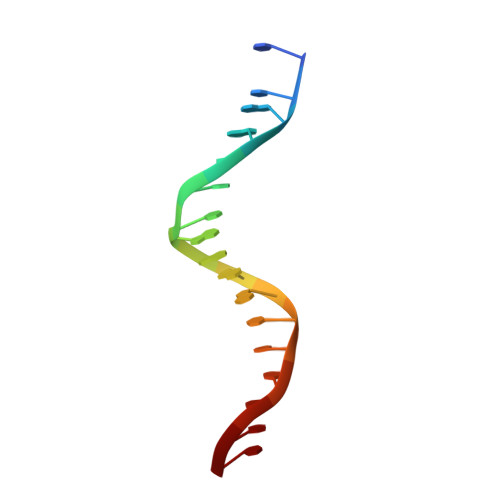
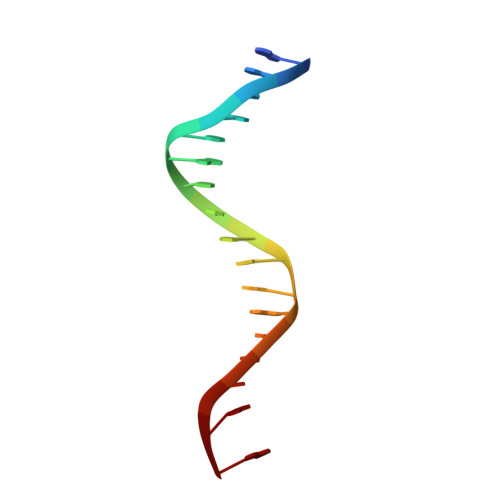
Modulation of RXR-DNA complex assembly by DNA context.
Osz, J., McEwen, A.G., Wolf, J., Poussin-Courmontagne, P., Peluso-Iltis, C., Chebaro, Y., Kieffer, B., Rochel, N.(2019) Mol Cell Endocrinol 481: 44-52
- PubMed: 30476562
- DOI: https://doi.org/10.1016/j.mce.2018.11.008
- Primary Citation of Related Structures:
6FBQ, 6FBR - PubMed Abstract:
Retinoid X Receptors (RXRs) act as dimer partners for several nuclear receptors including itself, binding to genomic DNA response elements and regulating gene transcription with cell and gene specificity. As homodimers, RXRs bind direct repeats of the half-site (A/G)G(G/T)TCA separated by 1 nucleotide (DR1) and little variability of this consensus site is observed for natural DR1s. However, these variations are responsible of the modulation of RXR receptors function through differential binding affinity and conformational changes. To further our understanding of the molecular mechanisms underlying RXR-DNA interactions, we examined how RXR DBDs bind to different DR1s using thermodynamics, X-ray crystallography and nuclear magnetic resonance (NMR) spectroscopy. We show that the half-site sequences modulate the binding cooperativity that results from the protein-protein contacts between the two DBDs. Chemical shifts perturbation NMR experiments revealed that sequence variations in half-sites induce changes that propagate from the protein-DNA interface to the dimerization interface throughout the DBD fold.
- Institut de Génétique et de Biologie Moléculaire et Cellulaire (IGBMC), Institut National de la Santé et de la Recherche Médicale (INSERM), U1258/Centre National de la Recherche Scientifique (CNRS), UMR7104/Université de Strasbourg, 67404, Illkirch, France.
Organizational Affiliation: