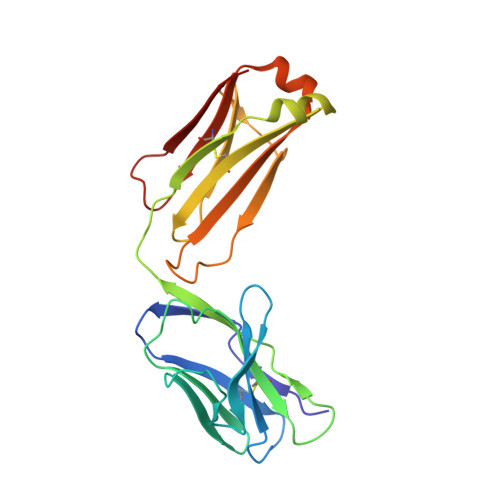
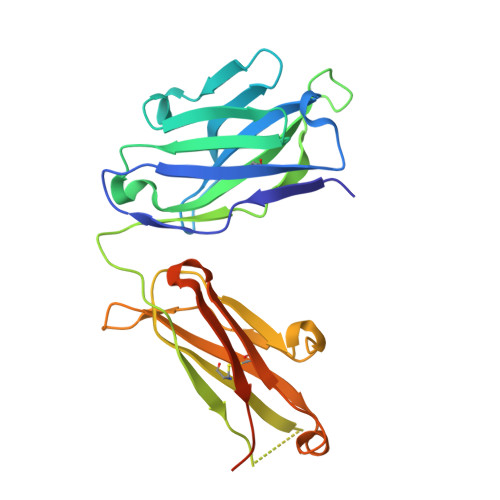
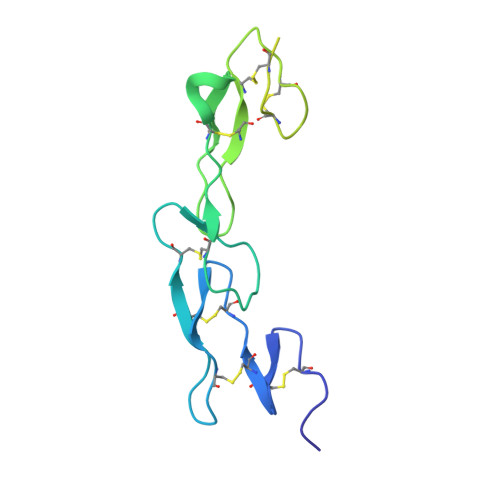
Complex Interplay between Epitope Specificity and Isotype Dictates the Biological Activity of Anti-human CD40 Antibodies.
Yu, X., Chan, H.T.C., Orr, C.M., Dadas, O., Booth, S.G., Dahal, L.N., Penfold, C.A., O'Brien, L., Mockridge, C.I., French, R.R., Duriez, P., Douglas, L.R., Pearson, A.R., Cragg, M.S., Tews, I., Glennie, M.J., White, A.L.(2018) Cancer Cell 33: 664-675.e4
- PubMed: 29576376
- DOI: https://doi.org/10.1016/j.ccell.2018.02.009
- Primary Citation of Related Structures:
6FAX - PubMed Abstract:
Anti-CD40 monoclonal antibodies (mAbs) that promote or inhibit receptor function hold promise as therapeutics for cancer and autoimmunity. Rules governing their diverse range of functions, however, are lacking. Here we determined characteristics of nine hCD40 mAbs engaging epitopes throughout the CD40 extracellular region expressed as varying isotypes. All mAb formats were strong agonists when hyper-crosslinked; however, only those binding the membrane-distal cysteine-rich domain 1 (CRD1) retained agonistic activity with physiological Fc gamma receptor crosslinking or as human immunoglobulin G2 isotype; agonistic activity decreased as epitopes drew closer to the membrane. In addition, all CRD2-4 binding mAbs blocked CD40 ligand interaction and were potent antagonists. Thus, the membrane distal CRD1 provides a region of choice for selecting CD40 agonists while CRD2-4 provides antagonistic epitopes.
- Antibody and Vaccine Group, Cancer Sciences Unit, University of Southampton Faculty of Medicine, Southampton SO16 6YD, UK.
Organizational Affiliation: