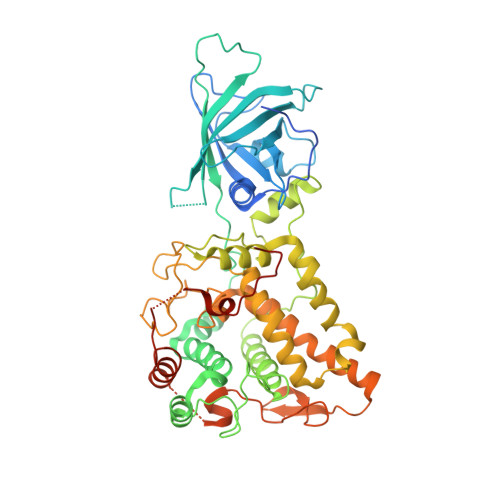
Pseudomonas aeruginosapyoverdine maturation enzyme PvdP has a noncanonical domain architecture and affords insight into a new subclass of tyrosinases.
Poppe, J., Reichelt, J., Blankenfeldt, W.(2018) J Biological Chem 293: 14926-14936
- PubMed: 30030378
- DOI: https://doi.org/10.1074/jbc.RA118.002560
- Primary Citation of Related Structures:
6EYS, 6EYV - PubMed Abstract:
Pyoverdines (PVDs) are important chromophore-containing siderophores of fluorescent pseudomonad bacteria such as the opportunistic human pathogen Pseudomonas aeruginosa in which they play an essential role in host infection. PVD biosynthesis encompasses a complex pathway comprising cytosolic nonribosomal peptide synthetases that produce a polypeptide precursor that periplasmic enzymes convert to the final product. The structures of most enzymes involved in PVD chromophore maturation have been elucidated, but the structure of the essential tyrosinase PvdP, a monooxygenase required for the penultimate step in PVD biosynthesis, is not known. Here, we closed this gap by determining the crystal structure of PvdP in an apo and tyrosine-complexed state at 2.1 and 2.7 Å, respectively. These structures revealed that PvdP is a homodimer, with each chain consisting of a C-terminal tyrosinase domain and an N-terminal eight-stranded β-barrel reminiscent of streptavidin that appears to have a structural role only. We observed that ligand binding leads to the displacement of a "placeholder" tyrosine that blocks the active site in the apo structure. This exposes a large, deep binding site that seems suitable for accommodating ferribactin, a substrate of PvdP in PVD biosynthesis. The binding site consists almost exclusively of residues from the tyrosinase domain. Of note, we also found that this domain is more closely related to tyrosinases from arthropods rather than to tyrosinases from other bacteria. In conclusion, our work unravels the structural basis of PvdP's activity in PVD biosynthesis, observations that may inform structure-guided development of PvdP-specific inhibitors to manage P. aeruginosa infections.
- From the Department Structure and Function of Proteins, Helmholtz Centre for Infection Research, Inhoffenstrasse 7, 38124 Braunschweig, Germany and.
Organizational Affiliation: