Crystal structure of 14-3-3 zeta in complex with ASK1 14-3-3 binding motif
Petrvalska, O., Lentini Santo, D., Obsilova, V., Obsil, T.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| 14-3-3 protein zeta/delta | 233 | Homo sapiens | Mutation(s): 0 Gene Names: YWHAZ | 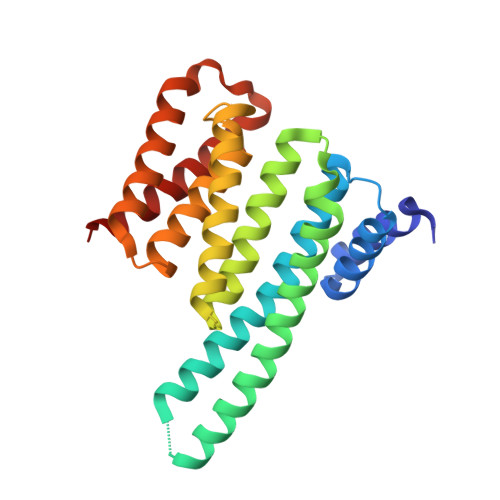 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P63104 (Homo sapiens) Explore P63104 Go to UniProtKB: P63104 | |||||
PHAROS: P63104 GTEx: ENSG00000164924 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P63104 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by: Sequence | 3D Structure
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Mitogen-activated protein kinase kinase kinase 5 | 8 | Homo sapiens | Mutation(s): 0 EC: 2.7.11.25 | 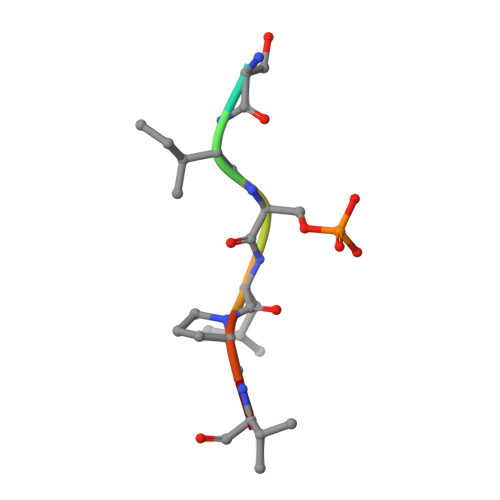 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q99683 (Homo sapiens) Explore Q99683 Go to UniProtKB: Q99683 | |||||
PHAROS: Q99683 GTEx: ENSG00000197442 | |||||
Entity Groups | |||||
| UniProt Group | Q99683 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| SEP Query on SEP | C, D | L-PEPTIDE LINKING | C3 H8 N O6 P |  | SER |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 74.98 | α = 90 |
| b = 93.427 | β = 99.74 |
| c = 68.491 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| XDS | data reduction |
| XDS | data scaling |
| MOLREP | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| European Commission | Czech Republic | 675179 - TASPPI - H2020-MSCA-ITN-2015 |