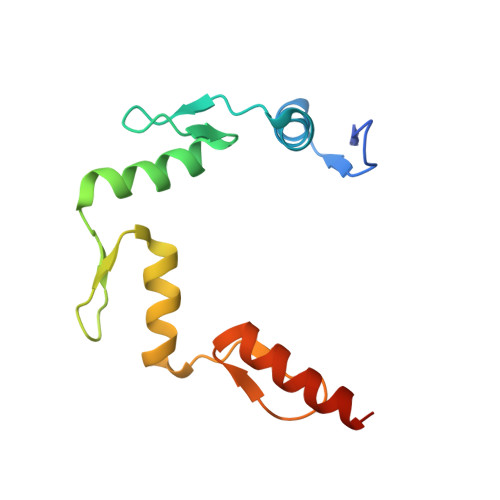
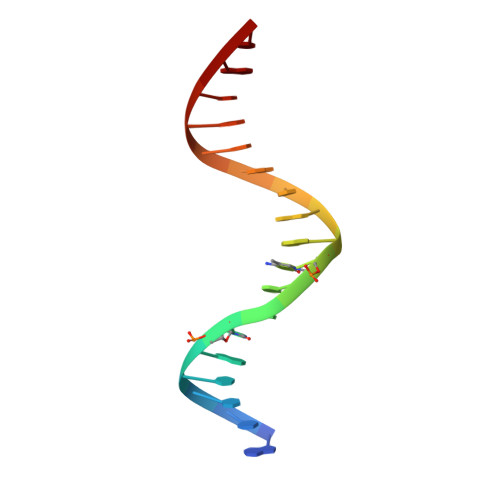
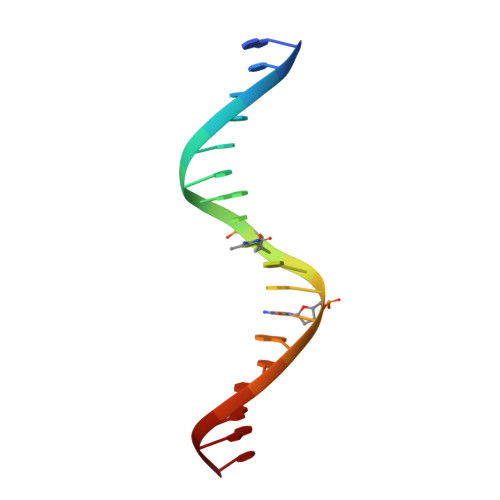
Structural insights into methylated DNA recognition by the C-terminal zinc fingers of the DNA reader protein ZBTB38.
Hudson, N.O., Whitby, F.G., Buck-Koehntop, B.A.(2018) J Biological Chem 293: 19835-19843
- PubMed: 30355731
- DOI: https://doi.org/10.1074/jbc.RA118.005147
- Primary Citation of Related Structures:
6E93, 6E94 - PubMed Abstract:
Methyl-CpG-binding proteins (MBPs) are selective readers of DNA methylation that play an essential role in mediating cellular transcription processes in both normal and diseased cells. This physiological function of MBPs has generated significant interest in understanding the mechanisms by which these proteins read and interpret DNA methylation signals. Zinc finger and BTB domain-containing 38 (ZBTB38) represents one member of the zinc finger (ZF) family of MBPs. We recently demonstrated that the C-terminal ZFs of ZBTB38 exhibit methyl-selective DNA binding within the ((A/G)TmCG(G/A)(mC/T)(G/A)) context both in vitro and within cells. Here we report the crystal structure of the first four C-terminal ZBTB38 ZFs (ZFs 6-9) in complex with the previously identified methylated consensus sequence at 1.75 Å resolution. From the structure, methyl-selective binding is preferentially localized at the 5' mCpG site of the bound DNA, which is facilitated through a series of base-specific interactions from residues within the α-helices of ZF7 and ZF8. ZF6 and ZF9 primarily stabilize ZF7 and ZF8 to facilitate the core base-specific interactions. Further structural and biochemical analyses, including solution NMR spectroscopy and electrophoretic mobility gel shift assays, revealed that the C-terminal ZFs of ZBTB38 utilize an alternative mode of mCpG recognition from the ZF MBPs structurally evaluated to date. Combined, these findings provide insight into the mechanism by which this ZF domain of ZBTB38 selectively recognizes methylated CpG sites and expands our understanding of how ZF-containing proteins can interpret this essential epigenetic mark.
- From the Departments of Chemistry and.
Organizational Affiliation: