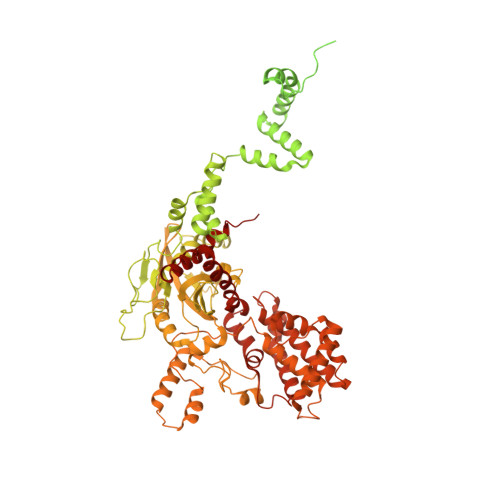
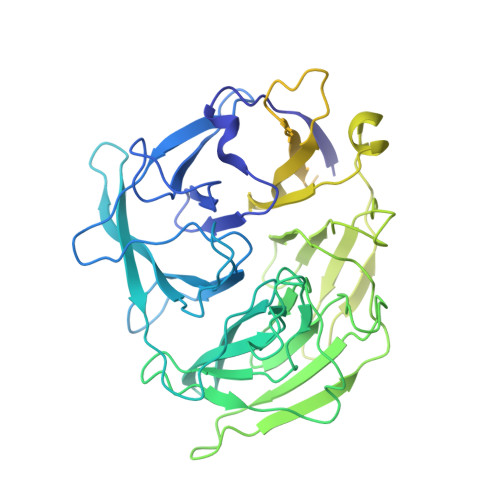
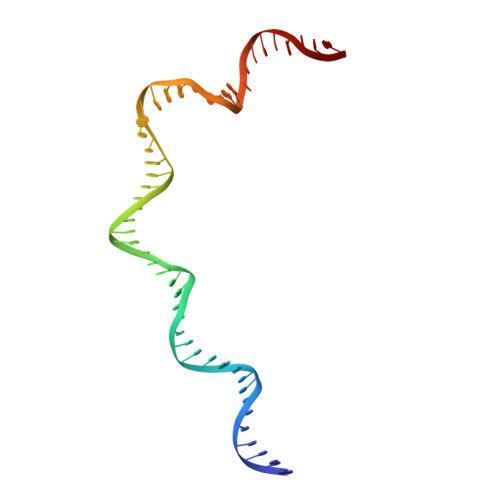
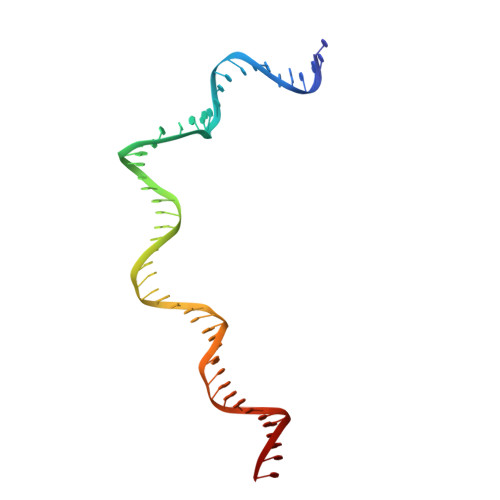
DNA melting initiates the RAG catalytic pathway.
Ru, H., Mi, W., Zhang, P., Alt, F.W., Schatz, D.G., Liao, M., Wu, H.(2018) Nat Struct Mol Biol 25: 732-742
- PubMed: 30061602
- DOI: https://doi.org/10.1038/s41594-018-0098-5
- Primary Citation of Related Structures:
6DBI, 6DBJ, 6DBL, 6DBO, 6DBQ, 6DBR, 6DBT, 6DBU, 6DBV, 6DBW, 6DBX - PubMed Abstract:
The mechanism for initiating DNA cleavage by DDE-family enzymes, including the RAG endonuclease, which initiates V(D)J recombination, is not well understood. Here we report six cryo-EM structures of zebrafish RAG in complex with one or two intact recombination signal sequences (RSSs), at up to 3.9-Å resolution. Unexpectedly, these structures reveal DNA melting at the heptamer of the RSSs, thus resulting in a corkscrew-like rotation of coding-flank DNA and the positioning of the scissile phosphate in the active site. Substrate binding is associated with dimer opening and a piston-like movement in RAG1, first outward to accommodate unmelted DNA and then inward to wedge melted DNA. These precleavage complexes show limited base-specific contacts of RAG at the conserved terminal CAC/GTG sequence of the heptamer, thus suggesting conservation based on a propensity to unwind. CA and TG overwhelmingly dominate terminal sequences in transposons and retrotransposons, thereby implicating a universal mechanism for DNA melting during the initiation of retroviral integration and DNA transposition.
- Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School, Boston, MA, USA.
Organizational Affiliation: