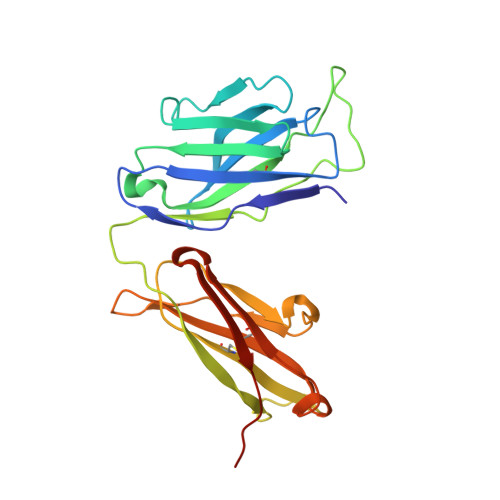
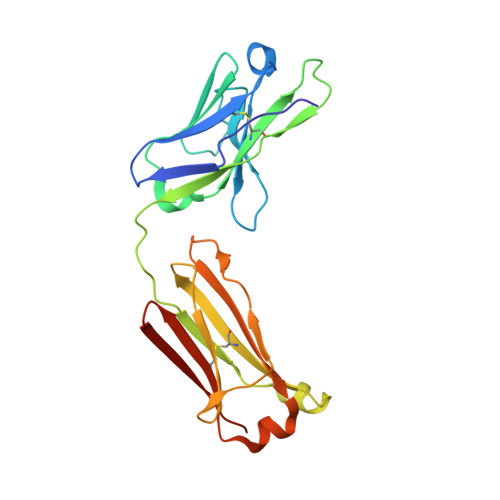
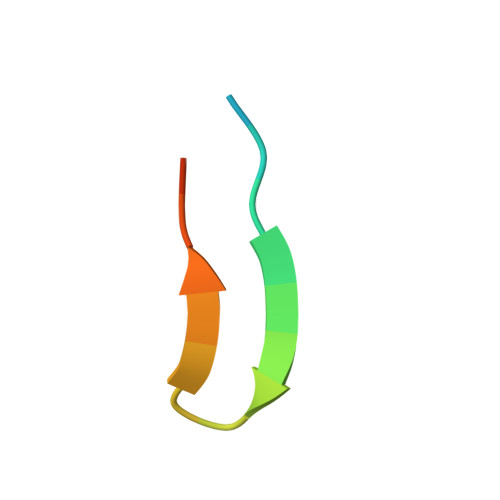
Structural Comparison of Human Anti-HIV-1 gp120 V3 Monoclonal Antibodies of the Same Gene Usage Induced by Vaccination and Chronic Infection.
Chan, K.W., Pan, R., Costa, M., Gorny, M.K., Wang, S., Lu, S., Kong, X.P.(2018) J Virol 92
- PubMed: 29997214
- DOI: https://doi.org/10.1128/JVI.00641-18
- Primary Citation of Related Structures:
6DB5, 6DB6, 6DB7 - PubMed Abstract:
Elucidating the structural basis of antibody (Ab) gene usage and affinity maturation of vaccine-induced Abs can inform the design of immunogens for inducing desired Ab responses in HIV vaccine development. Analyses of monoclonal Abs (MAbs) encoded by the same immunoglobulin genes at different stages of maturation can help to elucidate the maturation process. We have analyzed four human anti-V3 MAbs with the same VH1-3*01 and VL3-10*01 gene usage. Two MAbs, TA6 and TA7, were developed from a vaccinee in the HIV vaccine phase I trial DP6-001 with a polyvalent DNA prime/protein boost regimen, and two others, 311-11D and 1334, were developed from HIV-infected patients. The somatic hypermutation (SHM) rates in VH of vaccine-induced MAbs are lower than in chronic HIV infection-induced MAbs, while those in VL are comparable. Crystal structures of the antigen-binding fragments (Fabs) in complex with V3 peptides show that these MAbs bind the V3 epitope with a new cradle-binding mode and that the V3 β-hairpin lies along the antigen-binding groove, which consists of residues from both heavy and light chains. Residues conserved from the germ line sequences form specific binding pockets accommodating conserved structural elements of the V3 crown hairpin, predetermining the Ab gene selection, while somatically mutated residues create additional hydrogen bonds, electrostatic interactions, and van der Waals contacts, correlating with an increased binding affinity. Our data provide a unique example of germ line sequences determining the primordial antigen-binding sites and SHMs correlating with affinity maturation of Abs induced by vaccine and natural HIV infection. IMPORTANCE Understanding the structural basis of gene usage and affinity maturation for anti-HIV-1 antibodies may help vaccine design and development. Antibodies targeting the highly immunogenic third variable loop (V3) of HIV-1 gp120 provide a unique opportunity for detailed structural investigations. By comparing the sequences and structures of four anti-V3 MAbs at different stages of affinity maturation but of the same V gene usage, two induced by vaccination and another two by chronic infection, we provide a fine example of how germ line sequence determines the essential elements for epitope recognition and how affinity maturation improves the antibody's recognition of its epitope.
- Department of Biochemistry and Molecular Pharmacology, NYU School of Medicine, New York, New York, USA.
Organizational Affiliation: