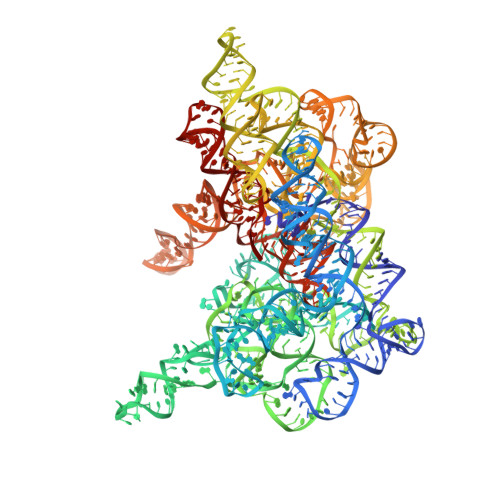
Structural basis for the second step of group II intron splicing.
Chan, R.T., Peters, J.K., Robart, A.R., Wiryaman, T., Rajashankar, K.R., Toor, N.(2018) Nat Commun 9: 4676-4676
- PubMed: 30410046
- DOI: https://doi.org/10.1038/s41467-018-06678-0
- Primary Citation of Related Structures:
6CHR, 6CIH - PubMed Abstract:
The group II intron and the spliceosome share a common active site architecture and are thought to be evolutionarily related. Here we report the 3.7 Å crystal structure of a eukaryotic group II intron in the lariat-3' exon form, immediately preceding the second step of splicing, analogous to the spliceosomal P complex. This structure reveals the location of the intact 3' splice site within the catalytic core of the group II intron. The 3'-OH of the 5' exon is positioned in close proximity to the 3' splice site for nucleophilic attack and exon ligation. The active site undergoes conformational rearrangements with the catalytic triplex having different configurations before and after the second step of splicing. We describe a complete model for the second step of group II intron splicing that incorporates a dynamic catalytic triplex being responsible for creating the binding pocket for 3' splice site capture.
- Department of Chemistry and Biochemistry, University of California, San Diego, La Jolla, CA, 92093, USA.
Organizational Affiliation: