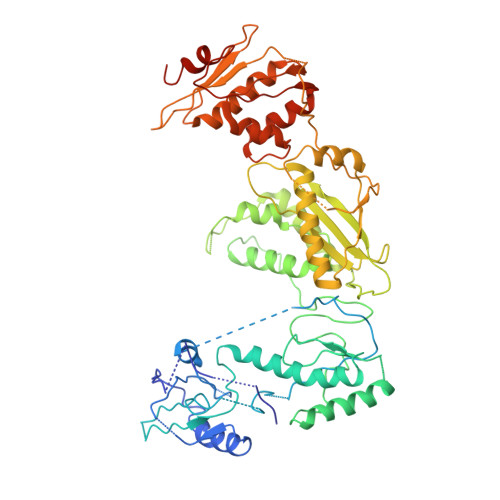
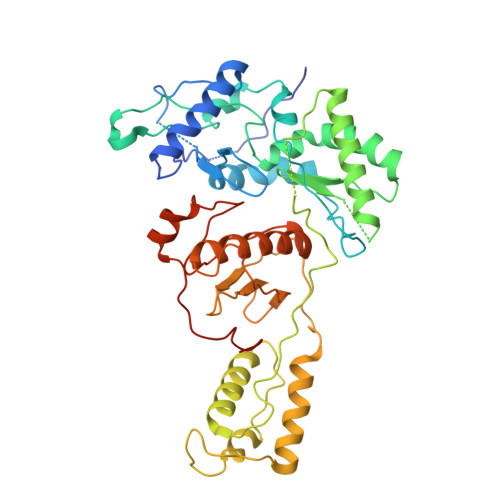
Architecture of an HIV-1 reverse transcriptase initiation complex.
Larsen, K.P., Mathiharan, Y.K., Kappel, K., Coey, A.T., Chen, D.H., Barrero, D., Madigan, L., Puglisi, J.D., Skiniotis, G., Puglisi, E.V.(2018) Nature 557: 118-122
- PubMed: 29695867
- DOI: https://doi.org/10.1038/s41586-018-0055-9
- Primary Citation of Related Structures:
6B19 - PubMed Abstract:
Reverse transcription of the HIV-1 RNA genome into double-stranded DNA is a central step in viral infection 1 and a common target of antiretroviral drugs 2 . The reaction is catalysed by viral reverse transcriptase (RT) 3,4 that is packaged in an infectious virion with two copies of viral genomic RNA 5 each bound to host lysine 3 transfer RNA (tRNA Lys 3 ), which acts as a primer for initiation of reverse transcription 6,7 . Upon viral entry into cells, initiation is slow and non-processive compared to elongation 8,9 . Despite extensive efforts, the structural basis of RT function during initiation has remained a mystery. Here we use cryo-electron microscopy to determine a three-dimensional structure of an HIV-1 RT initiation complex. In our structure, RT is in an inactive polymerase conformation with open fingers and thumb and with the nucleic acid primer-template complex shifted away from the active site. The primer binding site (PBS) helix formed between tRNA Lys 3 and HIV-1 RNA lies in the cleft of RT and is extended by additional pairing interactions. The 5' end of the tRNA refolds and stacks on the PBS to create a long helical structure, while the remaining viral RNA forms two helical stems positioned above the RT active site, with a linker that connects these helices to the RNase H region of the PBS. Our results illustrate how RNA structure in the initiation complex alters RT conformation to decrease activity, highlighting a potential target for drug action.
- Program in Biophysics, Stanford University, Stanford, CA, USA.
Organizational Affiliation: