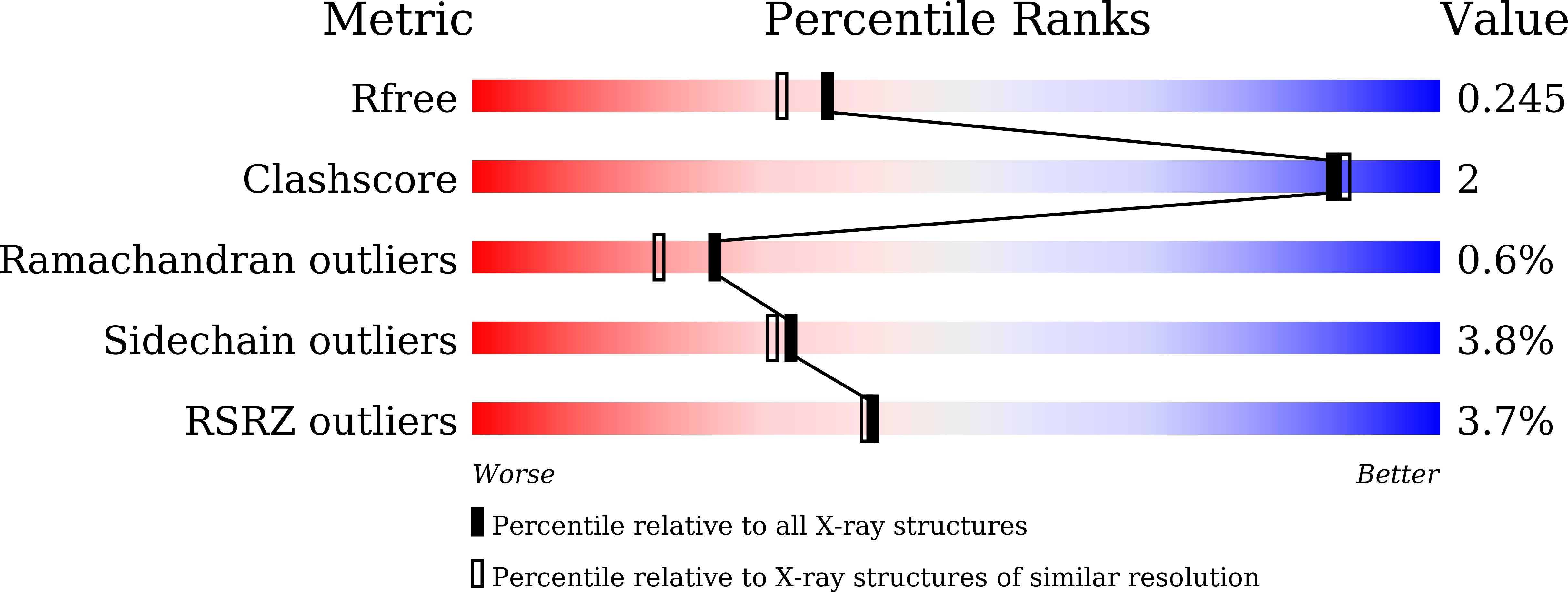
Discovery, Structural and Biochemical Studies of a rare Glu/Asp Specific M1 Class Aminopeptidase from Legionella pneumophila
Marapaka, A.K., Pillalamarri, V., Gumpena, R., Haque, N., Bala, S.C., Jangam, A., Addlagatta, A.(2018) Int J Biol Macromol 120: 1111-1118
- PubMed: 30172821
- DOI: https://doi.org/10.1016/j.ijbiomac.2018.08.172
- Primary Citation of Related Structures:
5ZI5, 5ZI7, 5ZIE - PubMed Abstract:
Aminopeptidases catalyze the hydrolysis of amino acids from the N-terminus of protein or peptide substrates. M1 family aminopeptidases are important for the pathogenicity of bacteria and play critical role in many physiological processes such as protein maturation, regulation of peptide hormone levels in humans. Most of the M1 family aminopeptidases reported till date display broad substrates specificity, mostly specific to basic and hydrophobic residues. In the current study we report the discovery of a novel M1 class aminopeptidase from Legionella pneumophila (LePepA), which cleaves only acidic residues. Biochemical and structural studies reveal that the S1 pocket is polar and positively charged. Bioinformatic analysis suggests that such active site is unique to only Legionella species and probably evolved for special needs of the microbe. Given its specific activity, LePepA could be useful in specific biotechnological applications.
Organizational Affiliation:
Applied Biology, CSIR-Indian Institute of Chemical Technology, Hyderabad, 500 007, Telangana, India; Academy of Scientific and Innovative Research (AcSIR), Rafi Marg, New Delhi 110001, India.