Structural and functional characterization of HIV-1 cell fusion inhibitor T20.
Zhang, X., Ding, X., Zhu, Y., Chong, H., Cui, S., He, J., Wang, X., He, Y.(2019) AIDS 33: 1-11
- PubMed: 30096076
- DOI: https://doi.org/10.1097/QAD.0000000000001979
- Primary Citation of Related Structures:
5ZCX - PubMed Abstract:
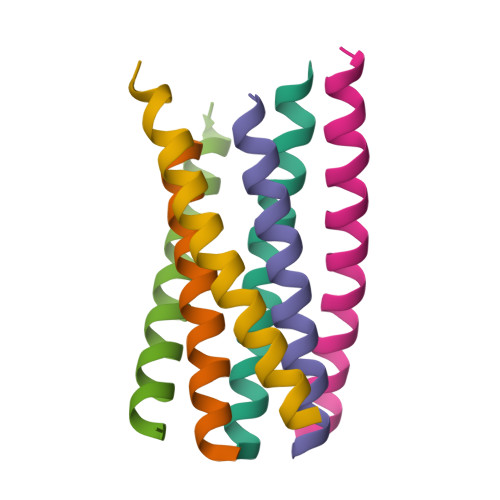
The peptide drug T20 (enfuvirtide), derived from the C-terminal heptad repeat region of HIV-1 gp41, is the only membrane fusion inhibitor available for treatment of viral infection; however, its mechanism of action remains elusive and its structural basis is lacking. We focused on determining the crystal structure of T20 in complex with N39, a target mimic peptide derived from the N-terminal heptad repeat region of gp41. On the basis of the structural information, the mechanisms of action of T20 and its resistance were further characterized. A panel of peptides was synthesized. The T20/N39 complex was assembled for crystallization studies. Circular dichroism spectroscopy, isothermal titration calorimetry (ITC), native polyacrylamide gel electrophoresis (N-PAGE), and mutational analysis were applied to analyze the structural and functional properties. A crystal structure of six-helical bundle (6-HB) structure formed by T20 and N39 was determined with a resolution limit of 2.3 Å, which revealed the critical intrahelical and interhelical interactions underlying the mechanism of action of T20 and its resistance mutations. Although the structural properties in the C-terminal tryptophan-rich motif (TRM) of T20 and the fusion peptide proximal region (FPPR) of N39 could not be finely defined by the structure, the data from biophysical and mutational analyses verified the essential roles of the TRM and FPPR motifs for the binding and inhibitory activities of T20. For the first time, our studies provide a structural basis of T20, which help our understanding on the mechanisms of HIV-1 fusion and its inhibition.
Organizational Affiliation:
MOH Key Laboratory of Systems Biology of Pathogens, Institute of Pathogen Biology and Center for AIDS Research, Chinese Academy of Medical Sciences and Peking Union Medical College.