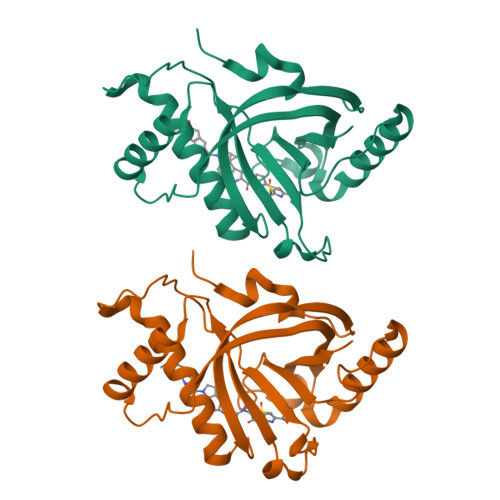
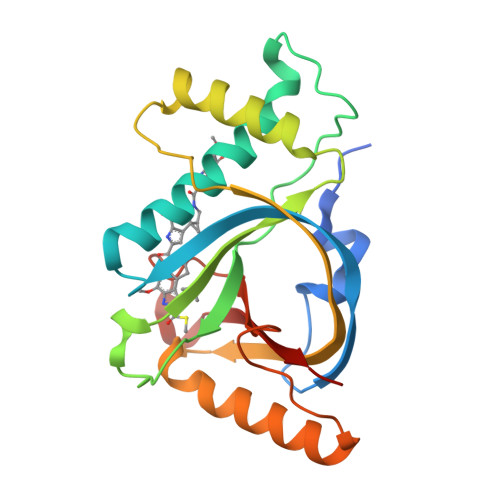
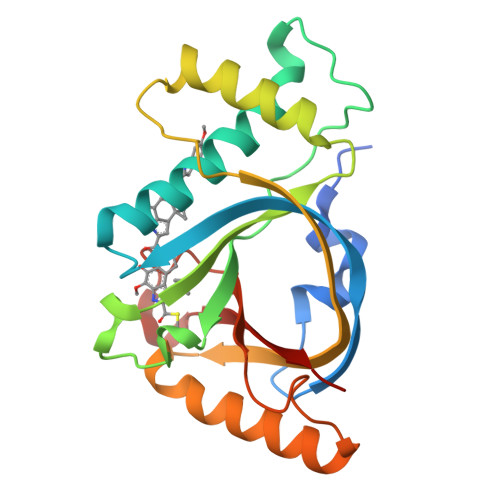
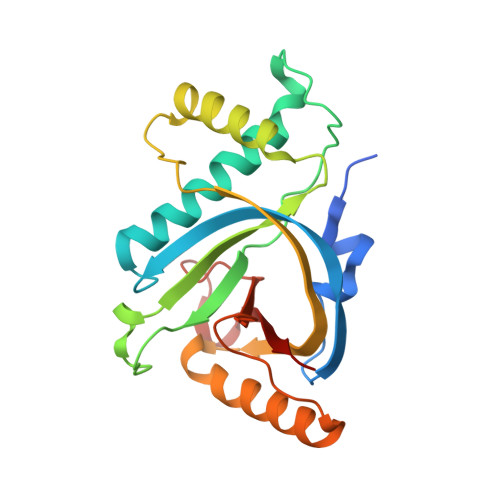
GyrI-like proteins catalyze cyclopropanoid hydrolysis to confer cellular protection
Yuan, H., Zhang, J., Cai, Y., Wu, S., Yang, K., Chan, H.C.S., Huang, W., Jin, W.B., Li, Y., Yin, Y., Igarashi, Y., Yuan, S., Zhou, J., Tang, G.L.(2017) Nat Commun 8: 1485-1485
- PubMed: 29133784
- DOI: https://doi.org/10.1038/s41467-017-01508-1
- Primary Citation of Related Structures:
5X5M - PubMed Abstract:
GyrI-like proteins are widely distributed in prokaryotes and eukaryotes, and recognized as small-molecule binding proteins. Here, we identify a subfamily of these proteins as cyclopropanoid cyclopropyl hydrolases (CCHs) that can catalyze the hydrolysis of the potent DNA-alkylating agents yatakemycin (YTM) and CC-1065. Co-crystallography and molecular dynamics simulation analyses reveal that these CCHs share a conserved aromatic cage for the hydrolytic activity. Subsequent cytotoxic assays confirm that CCHs are able to protect cells against YTM. Therefore, our findings suggest that the evolutionarily conserved GyrI-like proteins confer cellular protection against diverse xenobiotics via not only binding, but also catalysis.
Organizational Affiliation:
State Key Laboratory of Bio-organic and Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032, China.