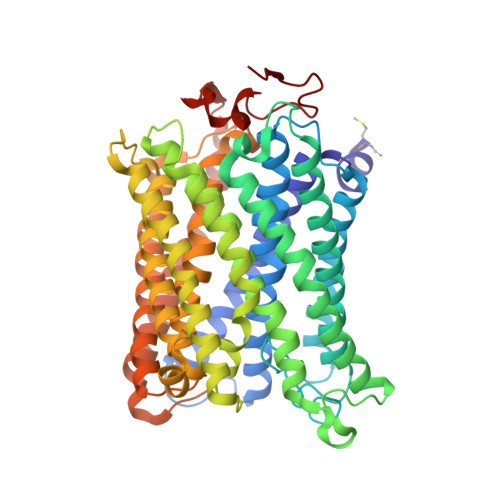
Crystal structure of CO-bound cytochrome c oxidase determined by serial femtosecond X-ray crystallography at room temperature.
Ishigami, I., Zatsepin, N.A., Hikita, M., Conrad, C.E., Nelson, G., Coe, J.D., Basu, S., Grant, T.D., Seaberg, M.H., Sierra, R.G., Hunter, M.S., Fromme, P., Fromme, R., Yeh, S.R., Rousseau, D.L.(2017) Proc Natl Acad Sci U S A 114: 8011-8016
- PubMed: 28698372
- DOI: https://doi.org/10.1073/pnas.1705628114
- Primary Citation of Related Structures:
5W97, 5WAU - PubMed Abstract:
Cytochrome c oxidase (C c O), the terminal enzyme in the electron transfer chain, translocates protons across the inner mitochondrial membrane by harnessing the free energy generated by the reduction of oxygen to water. Several redox-coupled proton translocation mechanisms have been proposed, but they lack confirmation, in part from the absence of reliable structural information due to radiation damage artifacts caused by the intense synchrotron radiation. Here we report the room temperature, neutral pH (6.8), damage-free structure of bovine C c O (bC c O) in the carbon monoxide (CO)-bound state at a resolution of 2.3 Å, obtained by serial femtosecond X-ray crystallography (SFX) with an X-ray free electron laser. As a comparison, an equivalent structure was obtained at a resolution of 1.95 Å, from data collected at a synchrotron light source. In the SFX structure, the CO is coordinated to the heme a 3 iron atom, with a bent Fe-C-O angle of ∼142°. In contrast, in the synchrotron structure, the Fe-CO bond is cleaved; CO relocates to a new site near Cu B , which, in turn, moves closer to the heme a 3 iron by ∼0.38 Å. Structural comparison reveals that ligand binding to the heme a 3 iron in the SFX structure is associated with an allosteric structural transition, involving partial unwinding of the helix-X between heme a and a 3 , thereby establishing a communication linkage between the two heme groups, setting the stage for proton translocation during the ensuing redox chemistry.
- Department of Physiology and Biophysics, Albert Einstein College of Medicine, Bronx, NY 10461.
Organizational Affiliation: