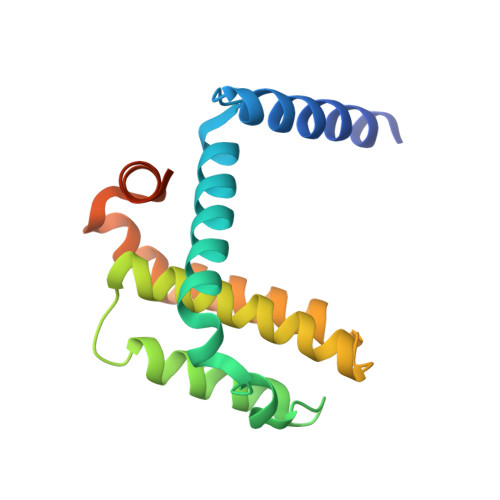
Conversion of Bim-BH3 from Activator to Inhibitor of Bak through Structure-Based Design.
Brouwer, J.M., Lan, P., Cowan, A.D., Bernardini, J.P., Birkinshaw, R.W., van Delft, M.F., Sleebs, B.E., Robin, A.Y., Wardak, A., Tan, I.K., Reljic, B., Lee, E.F., Fairlie, W.D., Call, M.J., Smith, B.J., Dewson, G., Lessene, G., Colman, P.M., Czabotar, P.E.(2017) Mol Cell 68: 659-672.e9
- PubMed: 29149594
- DOI: https://doi.org/10.1016/j.molcel.2017.11.001
- Primary Citation of Related Structures:
5VWV, 5VWW, 5VWX, 5VWY, 5VWZ, 5VX0, 5VX1, 5VX2, 5VX3 - PubMed Abstract:
Certain BH3-only proteins transiently bind and activate Bak and Bax, initiating their oligomerization and the permeabilization of the mitochondrial outer membrane, a pivotal step in the mitochondrial pathway to apoptosis. Here we describe the first crystal structures of an activator BH3 peptide bound to Bak and illustrate their use in the design of BH3 derivatives capable of inhibiting human Bak on mitochondria. These BH3 derivatives compete for the activation site at the canonical groove, are the first engineered inhibitors of Bak activation, and support the role of key conformational transitions associated with Bak activation.
Organizational Affiliation:
Walter and Eliza Hall Institute of Medical Research, 1G Royal Parade, Parkville, VIC 3052, Australia; Department of Medical Biology, The University of Melbourne, Melbourne, VIC 3052, Australia.