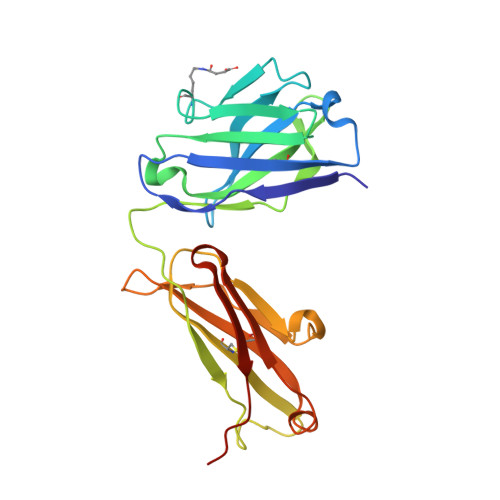
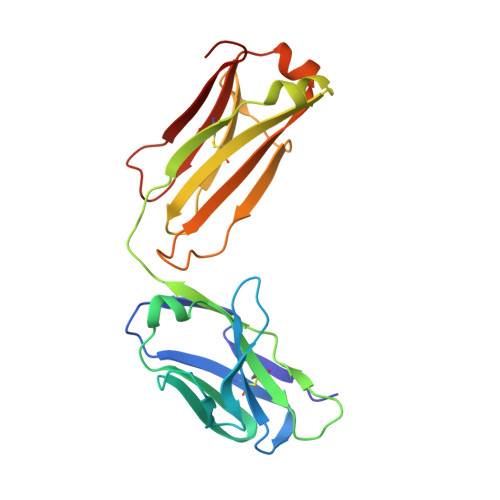
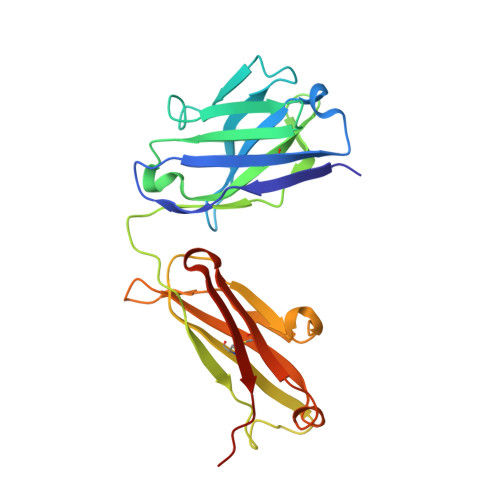
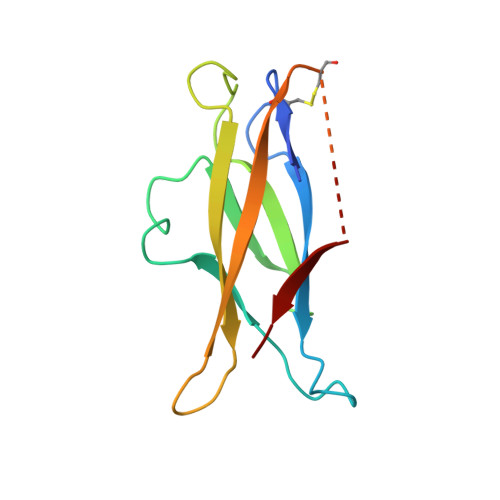
Discovery and Optimization of HKT288, a Cadherin-6-Targeting ADC for the Treatment of Ovarian and Renal Cancers.
Bialucha, C.U., Collins, S.D., Li, X., Saxena, P., Zhang, X., Durr, C., Lafont, B., Prieur, P., Shim, Y., Mosher, R., Lee, D., Ostrom, L., Hu, T., Bilic, S., Rajlic, I.L., Capka, V., Jiang, W., Wagner, J.P., Elliott, G., Veloso, A., Piel, J.C., Flaherty, M.M., Mansfield, K.G., Meseck, E.K., Rubic-Schneider, T., London, A.S., Tschantz, W.R., Kurz, M., Nguyen, D., Bourret, A., Meyer, M.J., Faris, J.E., Janatpour, M.J., Chan, V.W., Yoder, N.C., Catcott, K.C., McShea, M.A., Sun, X., Gao, H., Williams, J., Hofmann, F., Engelman, J.A., Ettenberg, S.A., Sellers, W.R., Lees, E.(2017) Cancer Discov 7: 1030-1045
- PubMed: 28526733
- DOI: https://doi.org/10.1158/2159-8290.CD-16-1414
- Primary Citation of Related Structures:
5VEB - PubMed Abstract:
Despite an improving therapeutic landscape, significant challenges remain in treating the majority of patients with advanced ovarian or renal cancer. We identified the cell-cell adhesion molecule cadherin-6 ( CDH6 ) as a lineage gene having significant differential expression in ovarian and kidney cancers. HKT288 is an optimized CDH6-targeting DM4-based antibody-drug conjugate (ADC) developed for the treatment of these diseases. Our study provides mechanistic evidence supporting the importance of linker choice for optimal antitumor activity and highlights CDH6 as an antigen for biotherapeutic development. To more robustly predict patient benefit of targeting CDH6, we incorporate a population-based patient-derived xenograft (PDX) clinical trial (PCT) to capture the heterogeneity of response across an unselected cohort of 30 models-a novel preclinical approach in ADC development. HKT288 induces durable tumor regressions of ovarian and renal cancer models in vivo , including 40% of models on the PCT, and features a preclinical safety profile supportive of progression toward clinical evaluation. Significance: We identify CDH6 as a target for biotherapeutics development and demonstrate how an integrated pharmacology strategy that incorporates mechanistic pharmacodynamics and toxicology studies provides a rich dataset for optimizing the therapeutic format. We highlight how a population-based PDX clinical trial and retrospective biomarker analysis can provide correlates of activity and response to guide initial patient selection for first-in-human trials of HKT288. Cancer Discov; 7(9); 1030-45. ©2017 AACR. This article is highlighted in the In This Issue feature, p. 920 .
- Novartis Institutes for Biomedical Research, Cambridge, Massachusetts. carl_uli.bialucha@novartis.com.
Organizational Affiliation: