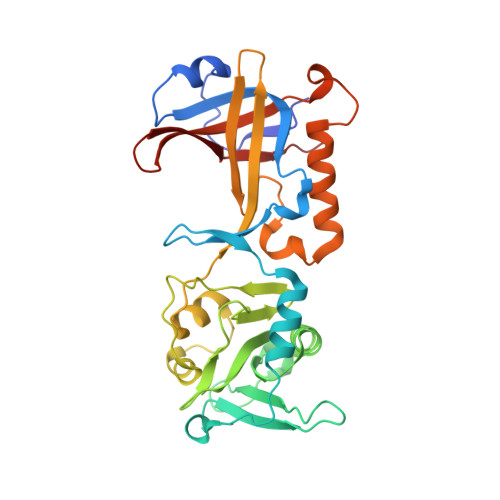
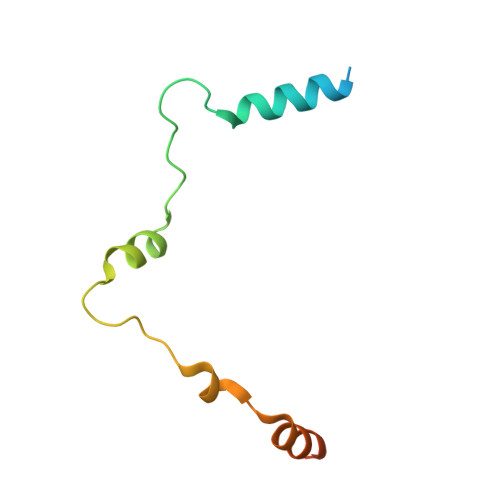
Structural and functional analysis of the human POT1-TPP1 telomeric complex.
Rice, C., Shastrula, P.K., Kossenkov, A.V., Hills, R., Baird, D.M., Showe, L.C., Doukov, T., Janicki, S., Skordalakes, E.(2017) Nat Commun 8: 14928-14928
- PubMed: 28393830
- DOI: https://doi.org/10.1038/ncomms14928
- Primary Citation of Related Structures:
5UN7 - PubMed Abstract:
POT1 and TPP1 are part of the shelterin complex and are essential for telomere length regulation and maintenance. Naturally occurring mutations of the telomeric POT1-TPP1 complex are implicated in familial glioma, melanoma and chronic lymphocytic leukaemia. Here we report the atomic structure of the interacting portion of the human telomeric POT1-TPP1 complex and suggest how several of these mutations contribute to malignant cancer. The POT1 C-terminus (POT1C) forms a bilobal structure consisting of an OB-fold and a holiday junction resolvase domain. TPP1 consists of several loops and helices involved in extensive interactions with POT1C. Biochemical data shows that several of the cancer-associated mutations, partially disrupt the POT1-TPP1 complex, which affects its ability to bind telomeric DNA efficiently. A defective POT1-TPP1 complex leads to longer and fragile telomeres, which in turn promotes genomic instability and cancer.
- The Wistar Institute, 3601 Spruce St, Philadelphia, Pennsylvania 19104, USA.
Organizational Affiliation: