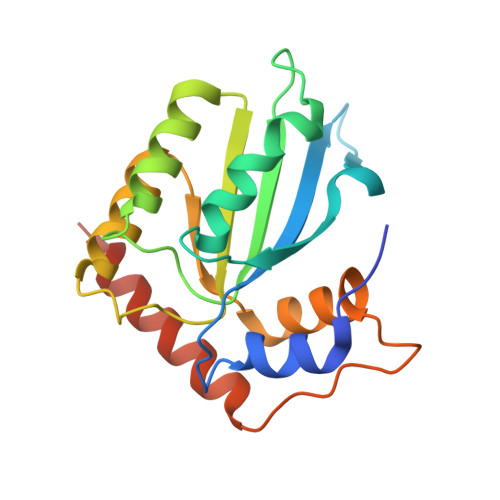
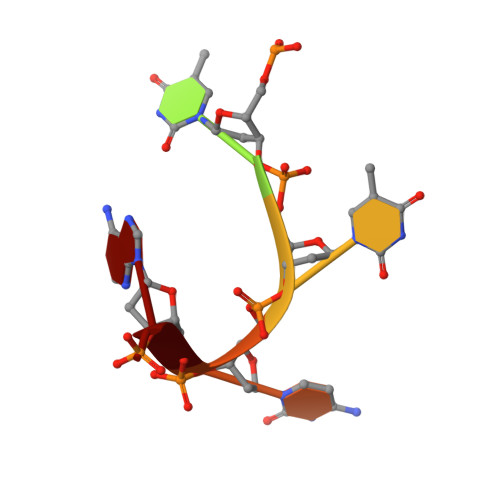
Structural basis for targeted DNA cytosine deamination and mutagenesis by APOBEC3A and APOBEC3B.
Shi, K., Carpenter, M.A., Banerjee, S., Shaban, N.M., Kurahashi, K., Salamango, D.J., McCann, J.L., Starrett, G.J., Duffy, J.V., Demir, O., Amaro, R.E., Harki, D.A., Harris, R.S., Aihara, H.(2017) Nat Struct Mol Biol 24: 131-139
- PubMed: 27991903
- DOI: https://doi.org/10.1038/nsmb.3344
- Primary Citation of Related Structures:
5SWW, 5TD5 - PubMed Abstract:
APOBEC-catalyzed cytosine-to-uracil deamination of single-stranded DNA (ssDNA) has beneficial functions in immunity and detrimental effects in cancer. APOBEC enzymes have intrinsic dinucleotide specificities that impart hallmark mutation signatures. Although numerous structures have been solved, mechanisms for global ssDNA recognition and local target-sequence selection remain unclear. Here we report crystal structures of human APOBEC3A and a chimera of human APOBEC3B and APOBEC3A bound to ssDNA at 3.1-Å and 1.7-Å resolution, respectively. These structures reveal a U-shaped DNA conformation, with the specificity-conferring -1 thymine flipped out and the target cytosine inserted deep into the zinc-coordinating active site pocket. The -1 thymine base fits into a groove between flexible loops and makes direct hydrogen bonds with the protein, accounting for the strong 5'-TC preference. These findings explain both conserved and unique properties among APOBEC family members, and they provide a basis for the rational design of inhibitors to impede the evolvability of viruses and tumors.
- Department of Biochemistry, Molecular Biology and Biophysics, University of Minnesota, Minneapolis, Minnesota, USA.
Organizational Affiliation: