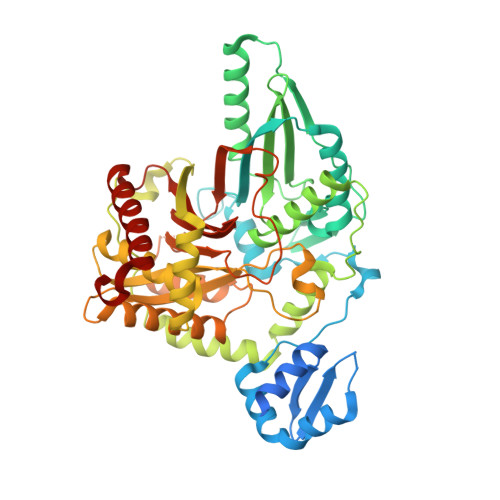
Structural elements of an NRPS cyclization domain and its intermodule docking domain.
Dowling, D.P., Kung, Y., Croft, A.K., Taghizadeh, K., Kelly, W.L., Walsh, C.T., Drennan, C.L.(2016) Proc Natl Acad Sci U S A 113: 12432-12437
- PubMed: 27791103
- DOI: https://doi.org/10.1073/pnas.1608615113
- Primary Citation of Related Structures:
5T7Z, 5T81 - PubMed Abstract:
Epothilones are thiazole-containing natural products with anticancer activity that are biosynthesized by polyketide synthase (PKS)-nonribosomal peptide synthetase (NRPS) enzymes EpoA-F. A cyclization domain of EpoB (Cy) assembles the thiazole functionality from an acetyl group and l-cysteine via condensation, cyclization, and dehydration. The PKS carrier protein of EpoA contributes the acetyl moiety, guided by a docking domain, whereas an NRPS EpoB carrier protein contributes l-cysteine. To visualize the structure of a cyclization domain with an accompanying docking domain, we solved a 2.03-Å resolution structure of this bidomain EpoB unit, comprising residues M1-Q497 (62 kDa) of the 160-kDa EpoB protein. We find that the N-terminal docking domain is connected to the V-shaped Cy domain by a 20-residue linker but otherwise makes no contacts to Cy. Molecular dynamic simulations and additional crystal structures reveal a high degree of flexibility for this docking domain, emphasizing the modular nature of the components of PKS-NRPS hybrid systems. These structures further reveal two 20-Å-long channels that run from distant sites on the Cy domain to the active site at the core of the enzyme, allowing two carrier proteins to dock with Cy and deliver their substrates simultaneously. Through mutagenesis and activity assays, catalytic residues N335 and D449 have been identified. Surprisingly, these residues do not map to the location of the conserved HHxxxDG motif in the structurally homologous NRPS condensation (C) domain. Thus, although both C and Cy domains have the same basic fold, their active sites appear distinct.
- Howard Hughes Medical Institute, Massachusetts Institute of Technology, Cambridge, MA 02139; daniel.dowling@umb.edu cdrennan@mit.edu.
Organizational Affiliation: