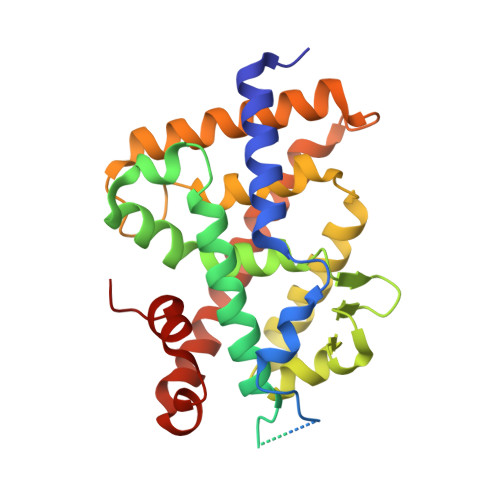
Structure-activity relationship study of vitamin D analogs with oxolane group in their side chain.
Belorusova, A.Y., Martinez, A., Gandara, Z., Gomez, G., Fall, Y., Rochel, N.(2017) Eur J Med Chem 134: 86-96
- PubMed: 28399453
- DOI: https://doi.org/10.1016/j.ejmech.2017.03.081
- Primary Citation of Related Structures:
5NKY, 5NMA, 5NMB - PubMed Abstract:
Synthetic analogs of 1α,25-dihydroxyvitamin D 3 (1,25(OH) 2 D 3 ) have been developed with the goal of improving the biological profile of the natural hormone for therapeutic applications. Derivatives of 1,25(OH) 2 D 3 with the oxolane moiety branched in the side chain at carbon C20, act as Vitamin D nuclear Receptor (VDR) superagonists being several orders of magnitude more active than the natural ligand. Here, we describe the synthesis and biological evaluation of three diastereoisomers of (1S, 3R)-Dihydroxy-(20S)-[(2″-hydroxy-2″-propyl)-tetrahydrofuryl]-22,23,24,25,26,27-hexanor-1α-hydroxyvitamin D3, with different stereochemistry at positions C2 and C5 of the oxolane ring branched at carbon C22 (1, C2RC5S; 2, C2SC5R; 3, C2SC5S). These compounds act as weak VDR agonist in transcriptional assays with compound 3 being the most active. X-ray crystallographic analysis of the VDR ligand-binding domain accommodating the three compounds indicates that the oxolane group branched at carbon C22 is not constrained as in case of compound with oxolane group branched at C20 leading to the loss of interactions of the triene group and increased flexibility of the C/D-rings and of the side chain.
- Department of Integrated Structural Biology, IGBMC (Institute of Genetics and of Molecular and Cellular Biology), 1 rue Laurent Fries, Illkirch, France; Centre National de la Recherche Scientifique (CNRS) UMR 7104, Illkirch, France; Institut National de la Santé et de la Recherche Médicale (INSERM) U964, Illkirch, France; Université de Strasbourg, Strasbourg, France.
Organizational Affiliation: