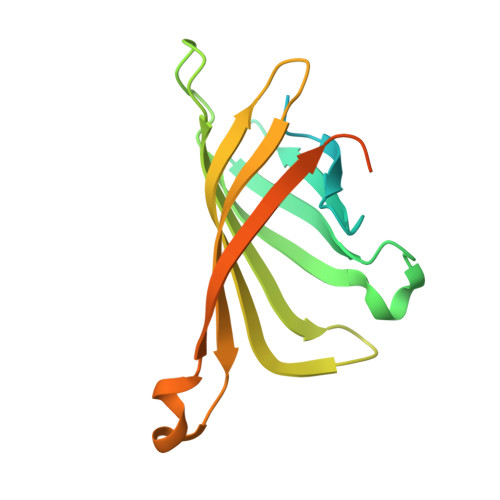
Stepwise Evolution Improves Identification of Diverse Peptides Binding to a Protein Target.
Lyamichev, V.I., Goodrich, L.E., Sullivan, E.H., Bannen, R.M., Benz, J., Albert, T.J., Patel, J.J.(2017) Sci Rep 7: 12116-12116
- PubMed: 28935886
- DOI: https://doi.org/10.1038/s41598-017-12440-1
- Primary Citation of Related Structures:
5N7X, 5N89, 5N8B, 5N8E, 5N8J, 5N8T, 5N8W, 5N99 - PubMed Abstract:
Considerable efforts have been made to develop technologies for selection of peptidic molecules that act as substrates or binders to a protein of interest. Here we demonstrate the combination of rational peptide array library design, parallel screening and stepwise evolution, to discover novel peptide hotspots. These hotspots can be systematically evolved to create high-affinity, high-specificity binding peptides to a protein target in a reproducible and digitally controlled process. The method can be applied to synthesize both linear and cyclic peptides, as well as peptides composed of natural and non-natural amino acid analogs, thereby enabling screens in a much diverse chemical space. We apply this method to stepwise evolve peptide binders to streptavidin, a protein studied for over two decades and report novel peptides that mimic key interactions of biotin to streptavidin.
- Roche Madison, 500 S Rosa Rd, Madison, WI, 53719, USA.
Organizational Affiliation: