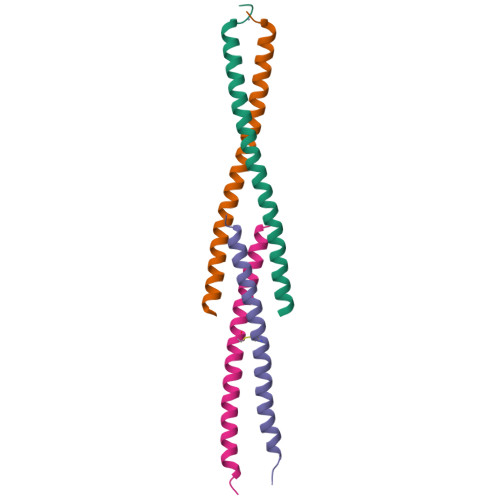Structural Basis for Mitotic Centrosome Assembly in Flies.
Feng, Z., Caballe, A., Wainman, A., Johnson, S., Haensele, A.F.M., Cottee, M.A., Conduit, P.T., Lea, S.M., Raff, J.W.(2017) Cell 169: 1078-1089.e13
- PubMed: 28575671
- DOI: https://doi.org/10.1016/j.cell.2017.05.030
- Primary Citation of Related Structures:
5I7C, 5MVW, 5MW0, 5MW9, 5MWE - PubMed Abstract:
In flies, Centrosomin (Cnn) forms a phosphorylation-dependent scaffold that recruits proteins to the mitotic centrosome, but how Cnn assembles into a scaffold is unclear. We show that scaffold assembly requires conserved leucine zipper (LZ) and Cnn-motif 2 (CM2) domains that co-assemble into a 2:2 complex in vitro. We solve the crystal structure of the LZ:CM2 complex, revealing that both proteins form helical dimers that assemble into an unusual tetramer. A slightly longer version of the LZ can form micron-scale structures with CM2, whose assembly is stimulated by Plk1 phosphorylation in vitro. Mutating individual residues that perturb LZ:CM2 tetramer assembly perturbs the formation of these micron-scale assemblies in vitro and Cnn-scaffold assembly in vivo. Thus, Cnn molecules have an intrinsic ability to form large, LZ:CM2-interaction-dependent assemblies that are critical for mitotic centrosome assembly. These studies provide the first atomic insight into a molecular interaction required for mitotic centrosome assembly.
Organizational Affiliation:
The Sir William Dunn School of Pathology, University of Oxford, South Parks Road, Oxford OX1 3RE, UK.