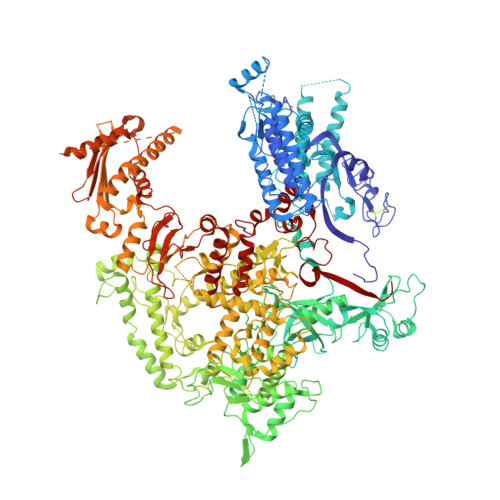
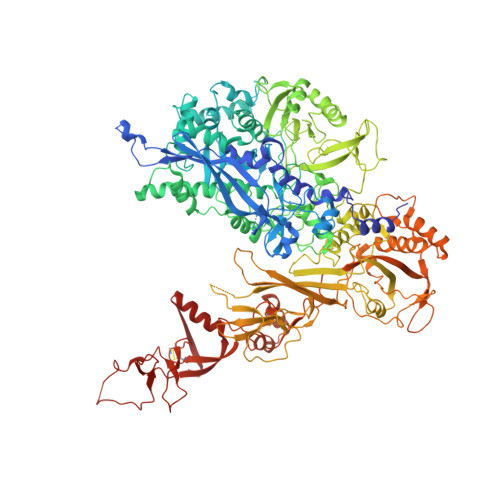
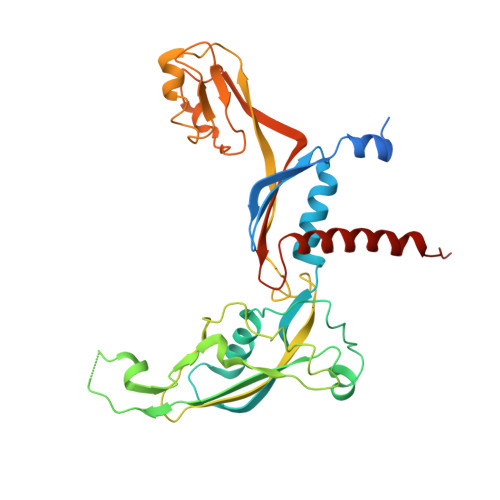
Molecular Structures of Transcribing RNA Polymerase I.
Tafur, L., Sadian, Y., Hoffmann, N.A., Jakobi, A.J., Wetzel, R., Hagen, W.J., Sachse, C., Muller, C.W.(2016) Mol Cell 64: 1135-1143
- PubMed: 27867008
- DOI: https://doi.org/10.1016/j.molcel.2016.11.013
- Primary Citation of Related Structures:
5M5W, 5M5X, 5M5Y, 5M64 - PubMed Abstract:
RNA polymerase I (Pol I) is a 14-subunit enzyme that solely synthesizes pre-ribosomal RNA. Recently, the crystal structure of apo Pol I gave unprecedented insight into its molecular architecture. Here, we present three cryo-EM structures of elongating Pol I, two at 4.0 Å and one at 4.6 Å resolution, and a Pol I open complex at 3.8 Å resolution. Two modules in Pol I mediate the narrowing of the DNA-binding cleft by closing the clamp domain. The DNA is bound by the clamp head and by the protrusion domain, allowing visualization of the upstream and downstream DNA duplexes in one of the elongation complexes. During formation of the Pol I elongation complex, the bridge helix progressively folds, while the A12.2 C-terminal domain is displaced from the active site. Our results reveal the conformational changes associated with elongation complex formation and provide additional insight into the Pol I transcription cycle.
- European Molecular Biology Laboratory (EMBL), Structural and Computational Biology Unit, Meyerhofstrasse 1, 69117 Heidelberg, Germany.
Organizational Affiliation: