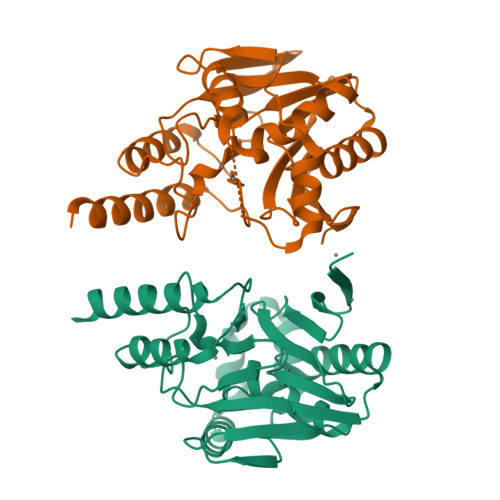
(19) F-NMR Reveals the Role of Mobile Loops in Product and Inhibitor Binding by the Sao Paulo Metallo-beta-Lactamase.
Abboud, M.I., Hinchliffe, P., Brem, J., Macsics, R., Pfeffer, I., Makena, A., Umland, K.D., Rydzik, A.M., Li, G.B., Spencer, J., Claridge, T.D., Schofield, C.J.(2017) Angew Chem Int Ed Engl 56: 3862-3866
- PubMed: 28252254
- DOI: https://doi.org/10.1002/anie.201612185
- Primary Citation of Related Structures:
5LS3 - PubMed Abstract:
Resistance to β-lactam antibiotics mediated by metallo-β-lactamases (MBLs) is a growing problem. We describe the use of protein-observe 19 F-NMR (PrOF NMR) to study the dynamics of the São Paulo MBL (SPM-1) from β-lactam-resistant Pseudomonas aeruginosa. Cysteinyl variants on the α3 and L3 regions, which flank the di-Zn II active site, were selectively 19 F-labeled using 3-bromo-1,1,1-trifluoroacetone. The PrOF NMR results reveal roles for the mobile α3 and L3 regions in the binding of both inhibitors and hydrolyzed β-lactam products to SPM-1. These results have implications for the mechanisms and inhibition of MBLs by β-lactams and non-β-lactams and illustrate the utility of PrOF NMR for efficiently analyzing metal chelation, identifying new binding modes, and studying protein binding from a mixture of equilibrating isomers.
Organizational Affiliation:
Department of Chemistry, University of Oxford, 12 Mansfield Road, OX1 3TA, Oxford, UK.