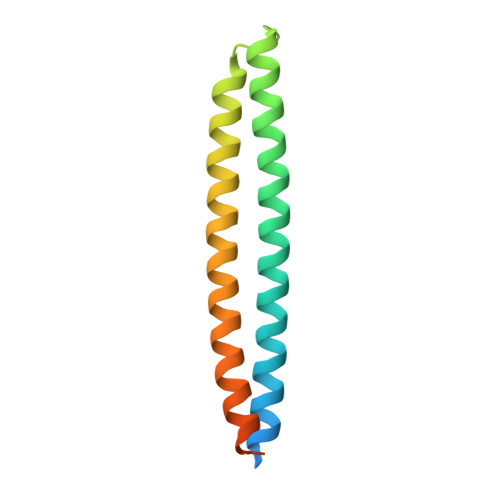
A secreted fungal histidine- and alanine-rich protein regulates metal ion homeostasis and oxidative stress.
Nostadt, R., Hilbert, M., Nizam, S., Rovenich, H., Wawra, S., Martin, J., Kupper, H., Mijovilovich, A., Ursinus, A., Langen, G., Hartmann, M.D., Lupas, A.N., Zuccaro, A.(2020) New Phytol 227: 1174-1188
- PubMed: 32285459
- DOI: https://doi.org/10.1111/nph.16606
- Primary Citation of Related Structures:
5LOS - PubMed Abstract:
Like pathogens, beneficial endophytic fungi secrete effector proteins to promote plant colonization, for example, through perturbation of host immunity. The genome of the root endophyte Serendipita indica encodes a novel family of highly similar, small alanine- and histidine-rich proteins, whose functions remain unknown. Members of this protein family carry an N-terminal signal peptide and a conserved C-terminal DELD motif. Here we report on the functional characterization of the plant-responsive DELD family protein Dld1 using a combination of structural, biochemical, biophysical and cytological analyses. The crystal structure of Dld1 shows an unusual, monomeric histidine zipper consisting of two antiparallel coiled-coil helices. Similar to other histidine-rich proteins, Dld1 displays varying affinity to different transition metal ions and undergoes metal ion- and pH-dependent unfolding. Transient expression of mCherry-tagged Dld1 in barley leaf and root tissue suggests that Dld1 localizes to the plant cell wall and accumulates at cell wall appositions during fungal penetration. Moreover, recombinant Dld1 enhances barley root colonization by S. indica, and inhibits H 2 O 2 -mediated radical polymerization of 3,3'-diaminobenzidine. Our data suggest that Dld1 has the potential to enhance micronutrient accessibility for the fungus and to interfere with oxidative stress and reactive oxygen species homeostasis to facilitate host colonization.
- Max Planck Institute for Terrestrial Microbiology, Karl-von-Frisch Str. 10, 35043, Marburg, Germany.
Organizational Affiliation: