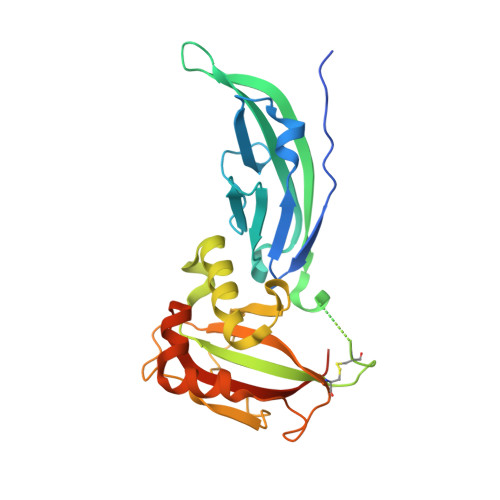
Structural flexibility of human alpha-dystroglycan.
Covaceuszach, S., Bozzi, M., Bigotti, M.G., Sciandra, F., Konarev, P.V., Brancaccio, A., Cassetta, A.(2017) FEBS Open Bio 7: 1064-1077
- PubMed: 28781947
- DOI: https://doi.org/10.1002/2211-5463.12259
- Primary Citation of Related Structures:
5LLK - PubMed Abstract:
Dystroglycan (DG), composed of α and β subunits, belongs to the dystrophin-associated glycoprotein complex. α-DG is an extracellular matrix protein that undergoes a complex post-translational glycosylation process. The bifunctional glycosyltransferase like-acetylglucosaminyltransferase (LARGE) plays a crucial role in the maturation of α-DG, enabling its binding to laminin. We have already structurally analyzed the N-terminal region of murine α-DG (α-DG-Nt) and of a pathological single point mutant that may affect recognition of LARGE, although the structural features of the potential interaction between LARGE and DG remain elusive. We now report on the crystal structure of the wild-type human α-DG-Nt that has allowed us to assess the reliability of our murine crystallographic structure as a α-DG-Nt general model. Moreover, we address for the first time both structures in solution. Interestingly, small-angle X-ray scattering (SAXS) reveals the existence of two main protein conformations ensembles. The predominant species is reminiscent of the crystal structure, while the less populated one assumes a more extended fold. A comparative analysis of the human and murine α-DG-Nt solution structures reveals that the two proteins share a common interdomain flexibility and population distribution of the two conformers. This is confirmed by the very similar stability displayed by the two orthologs as assessed by biochemical and biophysical experiments. These results highlight the need to take into account the molecular plasticity of α-DG-Nt in solution, as it can play an important role in the functional interactions with other binding partners.
- Istituto di Cristallografia CNR, Trieste Outstation Italy.
Organizational Affiliation: