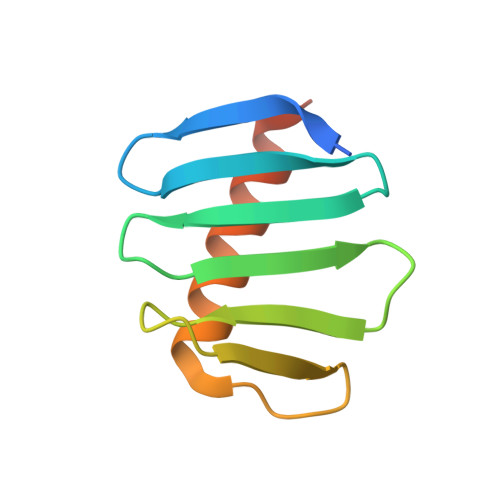
A key centriole assembly interaction interface between human PLK4 and STIL appears to not be conserved in flies.
Cottee, M.A., Johnson, S., Raff, J.W., Lea, S.M.(2017) Biol Open 6: 381-389
- PubMed: 28202467
- DOI: https://doi.org/10.1242/bio.024661
- Primary Citation of Related Structures:
5LHW, 5LHX, 5LHY, 5LHZ - PubMed Abstract:
A small number of proteins form a conserved pathway of centriole duplication. In humans and flies, the binding of PLK4/Sak to STIL/Ana2 initiates daughter centriole assembly. In humans, this interaction is mediated by an interaction between the Polo-Box-3 (PB3) domain of PLK4 and the coiled-coil domain of STIL (HsCCD). We showed previously that the Drosophila Ana2 coiled-coil domain (DmCCD) is essential for centriole assembly, but it forms a tight parallel tetramer in vitro that likely precludes an interaction with PB3. Here, we show that the isolated HsCCD and HsPB3 domains form a mixture of homo-multimers in vitro , but these readily dissociate when mixed to form the previously described 1:1 HsCCD:HsPB3 complex. In contrast, although Drosophila PB3 (DmPB3) adopts a canonical polo-box fold, it does not detectably interact with DmCCD in vitro Thus, surprisingly, a key centriole assembly interaction interface appears to differ between humans and flies.
- Sir William Dunn School of Pathology, University of Oxford, Oxford OX1 3RE, UK.
Organizational Affiliation: