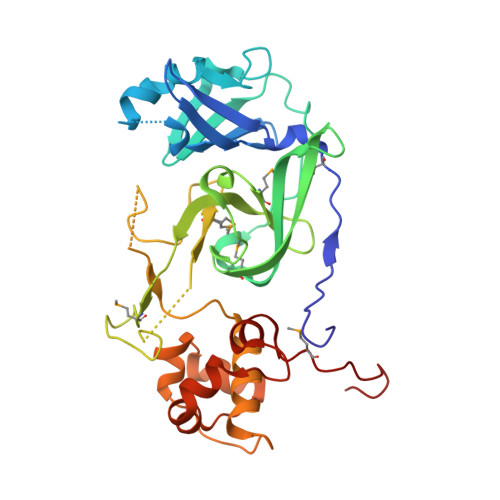
Structural basis for catalysis and substrate specificity of a 3C-like cysteine protease from a mosquito mesonivirus.
Kanitz, M., Blanck, S., Heine, A., Gulyaeva, A.A., Gorbalenya, A.E., Ziebuhr, J., Diederich, W.E.(2019) Virology 533: 21-33
- PubMed: 31078932
- DOI: https://doi.org/10.1016/j.virol.2019.05.001
- Primary Citation of Related Structures:
5LAC, 5LAK - PubMed Abstract:
Cavally virus (CavV) is a mosquito-borne plus-strand RNA virus in the family Mesoniviridae (order Nidovirales). We present X-ray structures for the CavV 3C-like protease (3CL pro ), as a free enzyme and in complex with a peptide aldehyde inhibitor mimicking the P4-to-P1 residues of a natural substrate. The 3CL pro structure (refined to 1.94 Å) shows that the protein forms dimers. The monomers are comprised of N-terminal domains I and II, which adopt a chymotrypsin-like fold, and a C-terminal α-helical domain III. The catalytic Cys-His dyad is assisted by a complex network of interactions involving a water molecule that mediates polar contacts between the catalytic His and a conserved Asp located in the domain II-III junction and is suitably positioned to stabilize the developing positive charge of the catalytic His in the transition state during catalysis. The study also reveals the structural basis for the distinct P2 Asn-specific substrate-binding pocket of mesonivirus 3CL pro s.
- Center for Tumor Biology and Immunology, Philipps University, Marburg, Germany; Institute of Pharmaceutical Chemistry, Philipps University, Marburg, Germany.
Organizational Affiliation: